Abstract
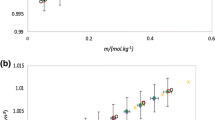
Density (ρ) and viscosity (η) of aqueous hippuric acid (HA) solutions containing LiCl and MnCl2 · 4H2O have been studied at 303.15 K in order to understand volumetric and viscometric behavior of these systems. Apparent molar volume (φv) of salts were calculated from density data and fitted to Massons relation and partial molar volumes (φ 0v ) at infinite dilution were determined. Relative viscosity data has been used to determine viscosity A and B coefficients using Jones-Dole relation. Partial molar volume and viscosity coefficients have been discussed in terms of ion-solvent interactions and overall structural fittings in solution.
Similar content being viewed by others
References
C. K. Rath, N. C. Rout, S. P. Das, and P. K. Mishra, Ultra Chem. 8, 205 (2012).
V. Kumar and R. Singh, Ultra Chem. 8, 361 (2012).
V. K. Syal, S. Chauhan, and M. S. Chauhan, Z. Phys. Chem. 159, 49 (1988).
M. N. Roy, R. Chanda, and R. K. Das, Phys. Chem. Liq., 1–22 (2011).
M. N. Roy, D. Nandi, and D. K. Hazra, J. Ind. Chem. Soc. 70, 121 (1993).
M. N. Roy, R. Chanda, R. K. Das, and D. Ekka, J. Chem. Eng. Data 56, 3285 (2011).
M. N. Roy, D. Ekka, and R. Dewan, Acta Chim. Slov. 58, 792 (2011).
P. C. Dey, M. A. Motin, T. K. Biswas, and E. M. Huque, Monatsh Chem. 134, 797 (2003).
M. J. Iqbal and M. A Chaudhry, J. Chem. Thermodyn. 41, 221 (2009).
S. J. Kharat, J. Mol. Liq. 140, 10 (2008).
M. N. Roy, A. Banerjee, and P. K. Roy, Int. J. Thermophys. 30, 515 (2009).
D. P. Gulwade, M. L. Narwade, and K. N. Wadodkar, Ind. J. Chem. A 43, 2102 (2004).
B. Sinha, V. K. Dakua, and M. N. Roy, J. Chem. Eng. Data 52, 1768 (2007).
A. F. Fucaloro, Y. Pu, K. Cha, A. Williams, and K. Conrad, J. Solut. Chem. 36, 61 (2007).
P. S. Nikam, R. P. Shewale, A. B. Sawant, and M. Hasan, J. Chem. Eng. Data 50, 487 (2005).
D. V. Jahagirdar, B. R. Arbad, C. S. Patil, and A. G. Shankarwar, Ind. J. Chem. A 40, 815 (2001).
J. Krakowiak, H. Koziel, and W. Grzybkowski, J. Mol. Liq. 118, 57 (2005).
A. N. Kannappan and R. Palani, Ind. J. Chem. A 46, 54 (2007).
S. D. Deosarkar, M. S. Menkudle, M. P. Shelke, and T. M. Kalyankar, Russ. J. Phys. Chem. A 88, 342 (2014).
S. D. Deosarkar, S. M. Deoraye, and T. M. Kalyankar, Russ. J. Phys. Chem. A 88, 1129 (2014).
S. D. Deosarkar and U. B. Shaikh, Russ. J. Gen. Chem. 83, 2392 (2013).
S. D. Deosarkar, Russ. J. Phys. Chem. A 87, 1420 (2013).
S. D. Deosarkar and M. L. Narwade, Russ. J. Phys. Chem. A 87, 1423 (2013).
S. D. Deosarkar and M. L. Narwade, Rasayan J. Chem. 3, 55 (2010).
S. D. Deosarkar, H. G. Jahagirdar, and V. B. Talwatkar, Rasayan J. Chem. 3, 755 (2010).
D. E. Henderson, Analytical Chemistry Laboratory Manual (Trinity College Hartford, 2006).
M. A. Saleh, S. Akhtar, M. S. Ahmed, and M. H. Uddin, Phys. Chem. Liq. 43, 367 (2005).
J. A. Dean, Lange’s Handbook of Chemistry, 15th ed. (McGraw-Hill, New York, 1999).
G. Jones and M. Dole, J. Am. Chem. Soc. 51, 2950 (1929).
R. Mathpal, B. K. Joshi, S. Joshi, and N. D. Kandpal, Monatsh. Chem. 137, 375 (2006).
B. Hawrylak, R. Palepu, and P. R. Tremaine, J. Chem. Thermodyn. 38, 988 (2006).
D. O. Masson, Philos Mag. 8, 218 (1929).
A. Ştefaniu, O. Iulian, and O. Ciocirlan, Rev. Roum. Chim. 56, 869 (2011).
B. Sinha, P. K. Roy, and M. N. Roy, Acta Chim. Slov. 57, 651 (2010).
P. S. Nikam and V. P. Sonawane, Int. J. Chem. Sci. 3, 2010 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Deosarkar, S.D., Tawde, P.D., Zinjade, A.B. et al. Partial molar volumes and viscosities of aqueous hippuric acid solutions containing LiCl and MnCl2 · 4H2O at 303.15 K. Russ. J. Phys. Chem. 89, 1556–1559 (2015). https://doi.org/10.1134/S0036024415090083
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415090083