Abstract
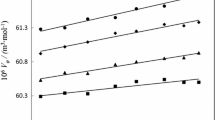
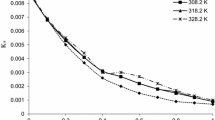
The enthalpies of solution and dilution of aqueous solutions of sodium ibuprofen (NaIBP) with concentrations of m < 1.4 mol/kg water are measured at 293.15, 298.15, 308.15, and 318.5 K using an isoperibolic calorimeter. The heat capacity of NaIBP in the temperature range of 273.15–528.15 K is measured using a DSC 204 F1 Phoenix differential scanning calorimeter (NETZSCH, Germany). The virial coefficients of the enthalpies of aqueous solutions of NaIBP are derived in terms of the Pitzer model, and the thermodynamic properties of both the solutions and the solution components are calculated over the range of compound solubility. The variation in these characteristics as a function of concentration and temperature is analyzed.
Similar content being viewed by others
References
H. J. Zimmerman, Semin. Liver Dis. 10, 322 (1990).
J. A. Mitchell and T. D. Warner, Nat. Rev. Drug Disc. 5, 75 (2006).
A. Fini, G. Fazio, and G. Feroci, Int. J. Pharm. 126, 95 (1995).
N. G. Manin, G. L. Perlovich, A. N. Manin, and A. Fini, Russ. J. Phys. Chem. A 81, 1062 (2007).
N. G. Manin, A. Fini, and G. L. Perlovich, J. Therm. Anal. Calorim. 104, 279 (2011).
N. G. Manin, G. L. Perlovich, and A. Fini, Russ. J. Phys. Chem. A 87, 580 (2013).
N. G. Manin, G. L. Perlovich, and A. Fini, Russ. J. Phys. Chem. A 88, 418 (2014).
N. G. Manin, A. Fini, and G. L. Perlovich, Russ. J. Phys. Chem. A 83, 187 (2009).
J. C. Moreno-Piraján, V. S. García-Cuello, and L. Giraldo-Gutierréz, E-J. Chem. 8, 1298 (2011).
R. Censi, V. Martena, E. Hoti, L. Malaj, and P. di Martino, J. Therm. Anal. Calorim. 111, 2009 (2013).
M. Bešter-Rogač, Acta Chim. Slov. 56, 70 (2009).
A. N. Kinchin, A. M. Kolker, and G. A. Krestov, Russ. J. Phys. Chem. A 60, 471 (1986).
Thermodynamic Properties of Individual Substances, The Handbook, Ed. by L. V. Gurvich, I. V. Veits, V. A. Medvedev, et al. (Nauka, Moscow, 1982; Hemisphere, New York, London, 1989), Vol. 4.
J. D. Cox and G. Pilcher, Thermochemistry of Organic and Organometallic Compounds (Academic Press, London (1970).
S. N. Solov’ev, N. M. Privalov, and A. F. Vorob’ev, Zh. Fiz. Khim. 50, 2719 (1976).
C. G. Malmberg and A. A. Moryott, J. Res. Nat. Bur. Stand. 56, 1 (1956).
K. S. Pitzer, J. Chem. Phys. 77, 268 (1973).
K. S. Pitzer, in Thermodynamic Modeling of Geological Materials: Minerals, Fluids and Melts, Ed. by I. S. E. Carmichael and H. P. Eugster, Reviews in Mineralogy (Mineralogic. Soc. of America, Washington DC, 1973), Vol. 17, p. 97.
L. F. Silvester and K. S. Pitzer, J. Phys. Chem. 81, 1822 (1977).
R. H. Busey, H. F. Holmes, and R. E. Mesmer, J. Chem. Thermodyn. 16, 343 (1984).
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Vysshaya Shkola, Moscow, 1982) [in Russian].
J. E. Mayrath and R. H. Wood, J. Chem. Thermodyn. 15, 625 (1983).
R. A. Robinson and R. H. Stokes, Electrolyte Solutions (Butterworths Scientific, London, 1959), p. 273.
A. V. Kustov and V. P. Korolev, Russ. J. Phys. Chem. A 80, 56 (2006).
V. P. Belousov and M. Yu. Panov, Thermodynamics of Aqueous Solutions of Nonelectrolytes (Khimiya, Leningrad, 1983) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.G. Manin, G.L. Perlovich, 2015, published in Zhurnal Fizicheskoi Khimii, 2015, Vol. 89, No. 4, pp. 658–667.
Rights and permissions
About this article
Cite this article
Manin, N.G., Perlovich, G.L. Thermodynamic properties of aqueous solutions of sodium ibuprofen at 293.15–318.15 K. Russ. J. Phys. Chem. 89, 644–653 (2015). https://doi.org/10.1134/S0036024415040160
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024415040160