Abstract
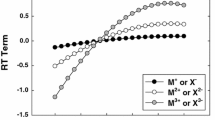
The concentration dependences of proton magnetic relaxation (PMR) rates measured at different temperatures in aqueous electrolyte solutions and concentrated seawater (SW) in a wide range of salt concentrations and for different seawater salinities are presented, along with the concentration dependences of PMR rates determined in salts dissolved directly in seawater. The coordination numbers of the basic ions in seawater were determined from the complete solvation limits and compared with those measured in single-component water-salt solutions. The attaining of complete solvation limits was determined using the PMR data for ions of different hydration signs.
Similar content being viewed by others
References
G. A. Krestov, Thermodynamics of Ionic Processes in Solutions (Khimiya, Leningrad, 1984) [in Russian].
V. K. Abrosimov, A. G. Krestov, et al., Achievements and Problems of the Solvation Theory: Structural and Thermodynamic Aspects (Nauka, Moscow, 1998) [in Russian].
The Water: Structure, State, Solvatation. Problems of Solution Chemistry, Ed. by A. M. Kutepov (Nauka, Moscow, 2003) [in Russian].
K. A. Valiev and M. N. Emel’yanova, J. Struct. Chem. 5, 625 (1964).
G. A. Krestov and V. K. Abrosimov, J. Struct. Chem. 8, 744 (1967).
E. A. Moelwin-Hughes, Physical Chemistry (Pergamon, London, New York, Paris, 1961; Inostr. Liter., Moscow, 1962).
R. D. Oparin, N. V. Fedotova, and V. N. Trostin, J. Struct. Chem. 43, 467 (2002).
M. N. Rodnikova, in Methods of Molecular Dynamics in Physical Chemistry (Nauka, Moscow, 1996), p. 235 [in Russian].
O. Ya. Samoilov, Structure of Aqueous Electrolytes and Hydration of Ions (Akad. Nauk SSSR, Moscow, 1957) [in Russian].
A. V. Teplukhin, Russ. Chem. Bull. 48, 842 (1999).
R. Horne, Marine Chemistry. The Structure of Water and the Chemistry of the Hydrosphere (Wiley-Interscience, New York, 1969).
T. Erdey-Gruz, Transport Phenomena in Aqueous Solutions (Wiley, New York, 1974; Mir, Moscow, 1976).
E. Berecz and M. Achs-Balla, Acta Chem. Acad. Sci. Hung. 73, 267 (1973).
N. A. Melnichenko, J. Struct. Chem. 48, 479 (2007).
N. A. Melnichenko, Russ. J. Phys. Chem. A 82, 1533 (2008).
N. A. Melnichenko, V. I. Chizhik, A. S. Vyskrebentsev, and A. V. Tyuveev, Russ. J. Phys. Chem. A 83, 1307 (2009).
V. I. Chizhik, Nuclear Magnetic Relaxation (Nauka, Leningrad, 1991) [in Russian].
N. A. Melnichenko, Russ. J. Phys. Chem. A 80, 1417 (2006).
A. V. Donets and V. I. Chizhik, Russ. J. Phys. Chem. A 79, 893 (2005).
N. A. Melnichenko and A. B. Slobodyuk, J. Glaciol. 59, 711 (2013).
N. A. Melnichenko, J. Glaciology 59(216), 719 (2013).
H. Cho, P. B. Shepson, L. A. Barrie, et al., J. Phys. Chem. 106, 11226 (2002).
Constants of Inorganic Substances, The Handbook, Ed. by R. A. Lidin, L. I. Andreeva, and V. A. Molochko (Drofa, Moscow, 2006) [in Russian].
F. J. Millero, F. Huang, and B. Graham, J. Solution Chem. 32, 473 (2003).
V. N. Afanas’ev and A. N. Ustinov, Russ. J. Inorg. Chem. 57, 1107 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.A. Melnichenko, 2014, published in Zhurnal Fizicheskoi Khimii, 2014, Vol. 88, No. 12, pp. 1932–1938.
Rights and permissions
About this article
Cite this article
Melnichenko, N.A. Ion hydration effects in aqueous solutions of strong electrolytes, according to proton magnetic relaxation measurements. Russ. J. Phys. Chem. 88, 2095–2101 (2014). https://doi.org/10.1134/S0036024414120218
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024414120218