Abstract
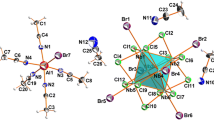
Trinuclear complexes Me4N[Ni3(µ3-F)(TFA)6(MeOH)2(H2O)] (1) and Me4N[M3(μ3-F)(TFA)6(py)3] (M = Mn (2), Co (3), Ni (4), Cu (5), Zn (6)) have been synthesized by crystallization from methanol solutions. Single-crystal X-ray diffraction shows that compounds 1–6 are composed of tetramethylammonium cations Me4N+ and trinuclear triangular anions [Ni3(µ3-F)(TFA)6(MeOH)2(H2O)]– (1) or [M3(μ3-F)(TFA)6(py)3]– (2–6) centered by the μ3-F atom. The bridging trifluoroacetate anions (TFA–) located along the triangle edges link pairs of M2+ cations, and the axial positions are occupied by MeOH, H2O, or pyridine (py) molecules. In 2, the pyridine molecules are nearly coplanar with the [M3F] triangle, while in the other structures they are turned almost perpendicularly. The different orientations of py molecules lead to different packing motifs: columns of alternating trinuclear anions and Me4N+ cations are formed in 2, while in 3–6 anions and cations form neutral layers. A significant role in the organization of structures 1–6 is played by non-covalent interactions, such as hydrogen bonds and stacking and CH···π interactions. Heating complexes 2–4 above 200°С turns out to lead to a stepwise thermal decomposition, which begins with the elimination of py and ends with the formation of d-metal fluoride above 300°С.





Similar content being viewed by others
REFERENCES
M. E. Nikiforova, I. A. Lutsenko, M. A. Kiskin, et al., Russ. J. Inorg. Chem. 66, 1343 (2021). https://doi.org/10.1134/S0036023621090102
V. V. Sharutin and O. K. Sharutina, Russ. J. Inorg. Chem. 66, 361 (2021). https://doi.org/10.1134/S0036023621030153
L. B. Serezhkina, D. S. Mitina, A. V. Vologzhanina, et al., Russ. J. Inorg. Chem. 67, 1769 (2022). https://doi.org/10.1134/S0036023622600915
T. Y. Chikineva, D. S. Koshelev, A. V. Medved’ko, et al., Russ. J. Inorg. Chem. 66, 170 (2021). https://doi.org/10.1134/S0036023621020054
D. S. Tereshchenko, I. V. Morozov, A. I. Boltalin, et al., Russ. J. Inorg. Chem. 49, 836 (2004).
D. S. Tereshchenko, I. V. Morozov, A. I. Boltalin, et al., Crystallogr. Rep. 58, 68 (2013). https://doi.org/10.1134/S106377451206017X
I. V. Morozov, E. V. Karpova, T. Yu. Glazunova, et al., Russ. J. Coord. Chem. 42, 647 (2016). https://doi.org/10.1134/S107032841610002X
Z.-L. Xie, M.-L. Feng, B. Tan, and X.-Y. Huang, Cryst-EngComm 14, 4894 (2012). https://doi.org/10.1039/C2CE25169H
J. Noack, C. Fritz, C. Flugel, et al., Dalton Trans. 42, 5706 (2013). https://doi.org/10.1039/c3dt32652g
J. P. S. Walsh, S. B. Meadows, A. Ghirri, et al., Inorg. Chem. 54, 12019 (2015). https://doi.org/10.1021/acs.inorgchem.5b01898
J. E. Reynolds III, K. M. Walsh, B. Li, et al., Chem. Commun. 54, 9937 (2018). https://doi.org/10.1039/C8CC05402A
D. Aulakh, T. Islamoglu, V. F. Bagundeset, et al., Chem. Mater. 30, 8332 (2018). https://doi.org/10.1021/acs.chemmater.8b03885
STOE WinXPOW, version 2.25, October 5, 2009, STOE & Cie GmbH.
Jana 2008. Version 25/10/2015, Vaclav Petricek, Michal Dusek, and Lukas Palatinus, Institute of Physics, Academy of Sciences of the Czech Republic (Praha).
PCPDFWIN. Version 2.2. June 2001. JCPDS-ICDD.
G. M. Sheldrick, Acta Cryst. A71, 3 (2015). https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick, C71, 3 (2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
R. D. Shannon, Acta Cryst. A32, 751 (1976). https://doi.org/10.1107/S0567739476001551
Cambridge Structural Database System (version 2019).
H. Irving and R. J. P. Williams, J. Chem. Soc. 3192 (1953). https://doi.org/10.1039/JR9530003192
T. Yu. Glazunova, D. S. Tereschenko, M. E. Buzoverov, et al., Russ. J. Coord. Chem. 47, 347 (2021). https://doi.org/10.1134/S1070328421040023
N. L. Armanasco, M. V. Baker, D. H. Brown, et al., Inorg. Chim. Acta 357, 4562 (2004). https://doi.org/10.1016/j.ica.2004.07.012
T. Steiner, Angew. Chem. Int. Ed. 41, 48 (2002). https://doi.org/10.1002/1521-3773(20020104)41:1%3C-48::AID-ANIE48%3E3.0.CO;2-U
J. A. K. Howard, V. J. Hoy, D. O’Hagan, and G. T. Smith, Tetrahedron 52, 12613 (1996). https://doi.org/10.1016/0040-4020(96)00749-1
T. Sierański, J. Mol. Model. 23, 338 (2017). https://doi.org/10.1007/s00894-017-3496-4
Y. Umezawa, S. Tsuboyama, K. Honda, et al., Bull. Chem. Soc. Jpn. 71, 1207 (1998). https://doi.org/10.1246/bcsj.71.1207
K. Shibasaki, A. Fujii, N. Mikami, and S. Tsuzuki, J. Phys. Chem. A 110, 4397 (2006). https://doi.org/10.1021/jp0605909
D. B. Kayumova, D. S. Tereschenko, T. B. Shatalova, et al., Russ. J. Coord. Chem. 48, 870 (2002). https://doi.org/10.1134/S1070328422700026
ACKNOWLEDGMENTS
The authors are grateful to the Shared Facility Center “Polymer Research Center” of the Institute of Synthetic Polymer Materials, RAS, with the support of the Ministry of Science and Higher Education of the Russian Federation (subject no. 0071-2021-0004) for the opportunity to conduct thermogravimetric analysis. The work used equipment purchased at the expense of the Development Program of the Moscow State University.
Funding
The study was supported by the Russian Science Foundation (project no. 22-72-10034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Supplementary Information
Rights and permissions
About this article
Cite this article
Tereshchenko, D.S., Buzoverov, M.E., Glazunova, T.Y. et al. A New Family of Trinuclear Complexes (CH3)4N[M3(µ3-F)(TFA)6(py)3] (M = Mn, Co, Ni, Cu, Zn): Synthesis, Crystal Structure, and Thermal Stability. Russ. J. Inorg. Chem. 68, 1282–1292 (2023). https://doi.org/10.1134/S0036023623601666
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023623601666