Abstract
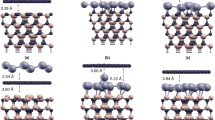
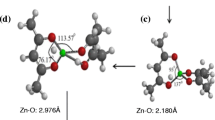
The processes of adsorption of a silicon oxide cluster onto the surface of reduced graphene oxide (GO) have been considered. The calculations have been performed at the PBE/PAW, ωB97XD/6-31G(d,p), and ωB97XD/6-31G(d,p)/6-311G(d,p)BSSE levels with inclusion of periodic conditions and in the cluster approximation. Upon the formation of GO, graphene sheets are distorted in the vicinity of bonding with oxygen. It is energetically favorable for the SinOx cluster to be located on the concave side of the surface (opposite of adsorbed oxygen). This stabilizes the position of the clusters and prevents the “drift” of silicon oxide during lithiation. The lithiation involves oxygen and silicon atoms. The lithium conductivity will depend on the Li/O and Li/Oc ratios, where O and Oc are the numbers of oxygen atoms on the silicon oxide and graphene oxide surfaces, respectively. Lithium migration occurs through oxygen atoms bound to silicon in the case of a small ratio Li/O ≤ 1/2 and captures Oc oxygen atoms covering graphene in the case of Li/O ≥ 1.



Similar content being viewed by others
REFERENCES
B. Kang and G. Ceder, Nature 458, 190 (2009). https://doi.org/10.1038/nature07853
H. Zhang and P. V. Braun, Nano Lett. 12, 2778 (2012). https://doi.org/10.1021/nl204551m
L. Qu, Y. Liu, J. -B. Baek, and L. Dai, ACS Nano 4, 1321 (2010). https://doi.org/10.1021/nn901850u
M. Giovanni, H. L. Poh, A. Ambrosi, et al., Nanoscale 4, 5002 (2012). https://doi.org/10.1039/C2NR31077E
Y. Tang, Z. Yang, and X. Dai, Phys. Chem. Chem. Phys. 14, 16566 (2012). https://doi.org/10.1039/C2CP41441D
Mehdi D. Esrafili, Fahimeh Sharifi, and Parisa Nematollahi, J. Mol. Graphics Model. 69, 8 (2016). https://doi.org/10.1016/j.jmgm.2016.08.005
T. Wehling, K. Novoselov, S. Morozov, et al., Nano Lett. 8, 173 (2008).
B. Guo, L. Fang, B. Zhang, and J. R. Gong, Insciences J. 1, 80 (2011). https://doi.org/10.5640/insc.010280
Y. Tang, J. Zhou, Z. Shen, et al., RSC Adv. 6, 93985 (2016). https://doi.org/10.1039/c6ra14476d
Y. Tang, W. Chen, Z. Shen, et al., Carbon 111, 448 (2017). https://doi.org/10.1016/j.carbon.2016.10.028
Y. Tang, Z. Liu, Z. Shen, et al., Sens. Actuators B: Chem. 238, 182 (2017). https://doi.org/10.1016/j.snb.2016.07.039
Y. Tang, Z. Shen, Y. Ma, et al., Mater. Chem. Phys. 207, 11 (2018). https://doi.org/10.1016/j.matchemphys.2017.12.048
Y. Tang, M. Zhang, W. Chen, et al., J. Phys. Chem. Solids 121, 247 (2018). https://doi.org/10.1016/j.jpcs.2018.05.037
Y. Tang, W. Chen, Z. Shen, et al., Phys. Chem. Chem. Phys. 20, 2284 (2018). https://doi.org/10.1039/C7CP07397F
G. Eda, G. Fanchini, and M. Chhowalla, Nat. Nanotechnol. 3, 270 (2008). https://doi.org/10.1038/nnano.2008.83
G. Lu, L. E. Ocola, and J. Chen, Nanotecnology 20, 445502 (2009). https://doi.org/10.1088/0957-4484/20/44/445502
D. R. Dreyer, S. Park, C. W. Bielawski, and R. S. Ruoff, Chem. Soc. Rev. 39, 228 (2010). https://doi.org/10.1039/B917103G
D. Chen, H. Feng, and J. Li, Chem. Rev. 112, 6027 (2012). https://doi.org/10.1021/cr300115g
K. Mkhoyan, A. Contryman, Silcox, et al., Microsc. Microanal. 16, 1704 (2010). https://doi.org/10.1017/s1431927610053961
Y. Zhu, S. Murali, W. Cai, et al., Adv. Mater. 22, 3906 (2010). https://doi.org/10.1002/adma.201001068
H. A. Becerril, J. Mao, Z. Liu, et al., ACS Nano 2, 463 (2008). https://doi.org/10.1021/nn700375n
D. Li, M. B. Mueller, S. Gilje, et al., Nature Nanotechnol. 3, 101 (2008). https://doi.org/10.1038/nnano.2007.451
G. Srinivas, J. W. Burress, J. Ford, and T. Yildirim, J. Mater. Chem. 21, 11323 (2011). https://doi.org/10.1039/c1jm11699a
Z. Pei, L. Li, L. Sun, et al., Carbon 51, 156 (2013). https://doi.org/10.1016/j.carbon.2012.08.024
L. Wang, K. Lee, Y. -Y. Sun, et al., ACS Nano 3, 2995 (2009). https://doi.org/10.1021/nn900667s
E. C. Mattson, K. Pande, M. Unger, et al., J. Phys. Chem. C 117, 10698 (2013). https://doi.org/10.1021/jp3122853
Y. Long, C. Zhang, X. Wang, et al., J. Mater. Chem. 21, 13934 (2011). https://doi.org/10.1039/c1jm12031j
H. He, J. Klinowski, M. Forster, and A. Lerf, Chem. Phys. Lett. 287, 53 (1998). https://doi.org/10.1016/s0009-2614(98)00144-4
A. Lerf, H. He, M. Forster, and J. Klinowski, J. Phys. Chem. B 102, 4477 (1998). https://doi.org/10.1021/jp9731821
M. D. Esrafili, F. Sharifi, and P. Nematollahi, J. Mol. Graphics Modell. 69, 8 (2016). https://doi.org/10.1016/j.jmgm.2016.08.005
M. D. Esrafili, ChemistrySelect 3, 12072 (2018).
B. Delley, J. Chem. Phys. 92, 508 (1990).
B. Delley, J. Chem. Phys. 113, 7756 (2000). https://doi.org/10.1063/1.1316015
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865 (1996). https://doi.org/10.1103/physrevlett.77.3865
S. Grimme, J. Comput. Chem. 27, 1787 (2006). https://doi.org/10.1002/jcc.20495
T. Szabo, O. Berkesi, P. Forgo, et al., Chem. Mater. 18, 2740 (2006). https://doi.org/10.1021/cm060258
L. A. Chernozatonskii, P. B. Sorokin, A. A. Artyukh, Russ. Chem. Rev. 83, 251 (2014). https://doi.org/10.1070/RC2014v083n03ABEH00436-7?locatt=label:RUS
K. A. Mkhoyan, A. W. Contryman, J. Silcox, et al., Nano Lett. 9, 1058 (2009). https://doi.org/10.1021/nl8034256
D. W. Boukhvalov and M. I. Katsnelson, J. Am. Chem. Soc. 130, 10697 (2008). https://doi.org/10.1021/ja8021686
M. D. Esrafili, F. Sharifi, and P. Nematollahi, Appl. Surf. Sci. 387, 454 (2016). https://doi.org/10.1016/j.apsusc.2016.06.127
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision A.02 (Gaussian, Inc., Wallingford, CT, 2009).
G. Kresse and J. Furthmuller, Phys. Rev. B 54, 11169 (1996). https://doi.org/10.1103/physrevb.54.11169
G. Kresse and D. Joubert, Phys. Rev. 59, 1758 (1999). https://doi.org/10.1103/physrevb.59.1758
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision B.01 (Gaussian, Inc., Wallingford CT, 2010). https://doi.org/10.1063/1.464906
J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys. 10 P, 6615 (2008). https://doi.org/10.1039/b810189b
A. V. Korchun, E. Yu. Evshchik, S. A. Baskakov, et al., Chim. Techno Acta 7, 259 (2020). https://doi.org/10.15826/chimtech.2020.7.4.21
D. Yu. Kornilova and S. P. Gubinb, Russ. J. Inorg. Chem. 65, 1965 (2020). https://doi.org/10.1134/S0036023620130021
N. Vats, S. Rauschenbach, W. Sigle, et al., Nanoscale 10, 4952 (2018). https://doi.org/10.1039/c8nr00402a
C. Gomez-Navarro, J. C. Meyer, R. S. Sundaram, et al., Nano Lett. 10, 1144 (2010). https://doi.org/10.1021/nl9031617
D. Y. Kornilov and L. A. Kasharina, Inorg. Mater. Appl. Res. 10, 1072 (2019). https://doi.org/10.1134/s2075113319050125
F. Perrozzi, S. Croce, E. Treossi, et al., Carbon 77, 473 (2014).
Funding
The study was performed at the Computing Center of the Institute of Problems of Chemical Physics, RAS, according to state assignment no. AAAA-A19-119061890019-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Zyubina, T.S., Zyubin, A.S., Korchun, A.V. et al. Lithiation of a Silicon Oxide Cluster Adsorbed onto Graphene Oxide: Quantum-Chemical Simulation. Russ. J. Inorg. Chem. 67, 1785–1793 (2022). https://doi.org/10.1134/S0036023622600708
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622600708