Abstract
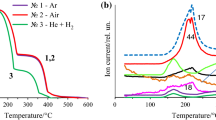
Information on the thermal decomposition of the double complex salt (DCS) [Cr(ur)6][Co(CN)6]⋅4Н2О (ur is urea CO(NH2)2) in the temperature range of 30–1000°C has been presented. The thermolysis of the studied DCS has been carried out in an argon atmosphere at three heating rates (5, 10, 20°C/min). The obtained TG data have been processed using the ARKS TA, and then the kinetics has been evaluated by the ARKS FK program from data for 5 and 10°C/min. The proposed multi-stage formal kinetic model provided a good fit of experimental data and showed a very reasonable prediction of decomposition at a rate of 20°C/min. It was demonstrated the formation of cyanobridge structures during thermolysis. A metastable compound Co3Cr was found in the product of thermolysis at 550°C. The mixture of the final products of calcination has been comprised Co0 (α-face-centered cubic lattice (fcc), β-fcc), Cr2O3, Cr7C3, Cr23C6, Cr21.26Co1.74C6.


Similar content being viewed by others
REFERENCES
R. Bala, D. Sachdeva, M. Kumar, et al., J. Coord. Chem. 73, 2801 (2020). https://doi.org/10.1080/00958972.2020.1836363
R. Bala, M. Kashyap, A. Kaur, et al., J. Mol. Struct. 1031, 246 (2013). https://doi.org/10.1016/j.molstruc.2012.09.080
R. Bala, M. Kashyap, A. Kaur, et al., Inorg. Chem. Commun. 29, 56 (2013). https://doi.org/10.1016/j.inoche.2012.12.001
D. Moon, S. Tanaka, T. Akitsu, et al., Acta Crystallogr., Sect. E Crystallogr. Commun. 71, 1336 (2015). https://doi.org/10.1107/S2056989015019258
E. S. Bazhina, M. A. Shmelev, A. A. Korlyukov, et al., Russ. J. Coord. Chem. 47, 105 (2021). https://doi.org/10.1134/S1070328421020019
E. S. Bazhina, M. A. Shmelev, M. A. Kiskin, et al., Russ. J. Coord. Chem. 47, 186 (2021). https://doi.org/10.1134/S1070328421030015
S. Pechenyuk, A. Zolotarev, Yu. Semushina, et al., Z. Krist. – Cryst. Mater. 233, 35 (2018). https://doi.org/10.1515/zkri-2016-2021
Y. P. Semushina, S. I. Pechenyuk, L. F. Kuzmich, et al., Russ. J. Phys. Chem. A. 91, 26 (2017). https://doi.org/10.1134/S003602441701023X
E. Y. Filatov, Y. P. Semushina, and A. N. Gosteva, J. Therm. Anal. Calorim. 134, 355 (2018). https://doi.org/10.1007/s10973-018-7230-y
D. P. Domonov and S. I. Pechenyuk, Russ. Chem. Bull. 67, 1041 (2018). https://doi.org/10.1007/s11172-018-2177-5
S. I. Pechenyuk, A. A. Zolotarev, A. N. Gosteva, et al., J. Mol. Struct. 1147, 388 (2017). https://doi.org/10.1016/j.molstruc.2017.06.099
A. N. Gosteva, P. E. Plyusnin, Y. P. Semushina, et al., J. Therm. Anal. Calorim. 134, 253 (2018). https://doi.org/10.1007/s10973-018-7428-z
A. N. Gosteva, D. P. Domonov, G. I. Kadyrova, et al., Izv. Vyss. Ucheb. Zaved. Khim. Khim. Tekhnol. 59, (2016). https://doi.org/10.6060/tcct.20165911.5380
S. V. Korenev, A. B. Venediktov, Y. V. Shubin, et al., ChemInform 35, (2004). https://doi.org/10.1002/chin.200417241
V. I. Lagunova, E. Yu. Filatov, P. E. Plusnin, et al., Russ. J. Inorg. Chem. 65, 1566 (2020). https://doi.org/10.1134/S0036023620100150
P. Kundu, S. Mitra, S. Kumar, et al., Transit. Met. Chem. 20, 417 (1995). https://doi.org/10.1007/BF00141508
T. Iguro, N. Ikeda, and T. Ohno, Inorg. Chim. Acta 226, 203 (1994). https://doi.org/10.1016/0020-1693(94)04088-5
P. Kundu, S. K. Dey, C. R. Choudhury, et al., Indian J. Chem., Sect. A 42, 1604 (2003).
G. Brauer, Handbuch der Präparativen Anorganischen Chemie: in Drei Bänden (Ferdinand Enke, Stuttgart, 1978).
http://www.cisp.spb.ru.
H. L. Friedman, J. Polym. Sci., Part C: Polym. Symp. 6, 183 (2007). https://doi.org/10.1002/polc.5070060121
A. Kossoy and Y. Akhmetshin, Process Saf. Prog. 26, 209 (2007). https://doi.org/10.1002/prs.10189
K. P. Gupta, J. Phase Equilibria Diffus. 27, 173 (2006). https://doi.org/10.1361/154770306X97588
D. P. Domonov, S. I. Pechenyuk, A. T. Belyaevskii, et al., Nanomaterials 10, 389 (2020). https://doi.org/10.3390/nano10020389
Y. Sakabe and H. Ogura, Anal. Sci. 8, 63 (1992). https://doi.org/10.2116/analsci.8.63
K. Nakomoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds: Part A: Theory and Applications in Inorganic Chemistry, 6th Ed. (John Wiley & Sons, Inc., 2008). https://doi.org/10.1002/9780470405840
E. Siebel, R. D. Fischer, N. A. Davies, et al., J. Organomet. Chem. 604, 34 (2000). https://doi.org/10.1016/S0022-328X(00)00198-4
M. Ferbinteanu, S. Tanase, M. Andruh, et al., Polyhedron 18, 3019 (1999). https://doi.org/10.1016/S0277-5387(99)00218-1
X.-Y. Liu, L.-Q. Duan, Q. Wei, et al., Inorganica Chim. Acta 423, 462 (2014). https://doi.org/10.1016/j.ica.2014.09.009
F. H. O. Ishiruji, N. L. Speziali, M. G. F. Vaz, et al., J. Braz. Chem. Soc. 21, 1195 (2010). https://doi.org/10.1590/S0103-50532010000700006
ACKNOWLEDGMENTS
The authors are very grateful to N.F. Sklokina for the X‑ray diffraction analysis and G.I. Kadyrova for the IR analysis of compounds.
Funding
This work was financially supported by Grant of President of Russian Federation (МК-5323.2021.1.3), and has been carried out in the framework of Scientific Research Contracts no. 0186-2021-0026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Gosteva, A., Kossoy, A., Tsvetov, N. et al. Dynamics of Thermal Decomposition of the Double Complex Salt [Cr(ur)6][Co(CN)6]⋅4Н2О. Russ. J. Inorg. Chem. 67, 1257–1263 (2022). https://doi.org/10.1134/S0036023622080150
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622080150