Abstract
A relatively low-temperature molten salt assisted synthesis is developed to prepare MAX phase solid solutions in the Ti2AlC–V2AlC system, to be used as precursors for the preparation of heterometallic MXenes Ti2 – xVxC. When the synthesis temperature is 1000°С, Ti1V1AlC, Ti0.5V1.5AlC, and Ti0.2V1.8AlC samples (despite the formation of a MAX phase and a systematic shift of reflection (002) in response to progressing vanadium-for-titanium substitution) typically feature appreciable amounts of impurity phases, namely Al2O3 and vanadium (their reflections are partially overlapping). An increase in synthetic temperature to 1100°С appreciably reduces the amount of impurities and, in a Ti1V1AlC sample, decreases the unit cell parameter a, which yet far exceeds the calculated and published values (while the parameter c corresponds with those values). Thermal analysis of Ti2AlC, Ti1V1AlC, and V2AlC samples in the range 20–1000°С shows that the oxidation resistance decreases in the progress of vanadium-for-titanium substitution in a MAX phase. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are used to study the microstructure of the prepared Ti2AlC and V2AlC phases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Although MAX phases were discovered in carbide and nitride systems as long ago as in the middle of the last century during the study of refractory systems [1–5], a new wave of scientific interest in them has been observed in recent years [6–12]. The prior attention to these compounds was as to components of refractory ceramic materials that could improve crack resistance and thermal shock resistance owing to their structural features [13, 14], namely, layerness (for example, in M2 + xAl(Si)Cx phases) arising from large spacings between M2 + xCx carbide layers interlayered by aluminum atoms; cracks are deviated while propagating to lose energy. One more important aspect is an option to improve the oxidation resistance and mechanical properties of ZrB2/HfB2 UHT ceramic materials [15–19], which are extremely promising for use in oxygen-containing environments at temperatures above 2000–2500°C [20–25].
Now MAX phases are used as precursor materials for manufacturing new 2D nanomaterials, namely, MXenes M2 + xCxTy [6, 7, 26, 27], which are predicted to be used very widely as, e.g., components of sensor materials [28–33], current sources, and supercapacitors [26, 34–36], in various biomedical applications [27, 37–39], and in other fields.
Therefore, the task arose of developing most cost-effective and reproducible methods for the synthesis of sufficiently large batches of precursors, that is, MAX phases with a tailored ratio of transition metals, which can be crucially important for the properties of MXenes based on them.
It is well known that the preparation of phase-pure MAX phase samples is a difficult task, for they usually contain impurities of transition-metal carbides, silicides (in the case of M2 + xSiCx), or intermetallics with aluminum (in the case of M2 + xAlCx), and oxides of all metals of the system involved. In the context of the subsequent preparation of MXenes, an important requirement is to minimize the content in the MAX phases of individual metal carbides and transition-metal oxides (removal of which can affect the in-plane stoichiometry of the thus-prepared MXenes M2 + xCxTy).
The most popular preparation of MAX phases is via hot pressing or cold pressing followed by sintering at sufficiently high temperatures (~1400–1500°C) [40–42]. In addition, hot pressing or spark plasma sintering most often uses graphite molds, due to which an additional carburization of the system is possible and, accordingly, the formation or an increase in the content of MC impurity phases can occur. Therefore, it is necessary that an excess of aluminum be used in the synthesis and a deficit of carbon or metal carbides in the precursor systems.
One of the simplest, energy efficient, and affordable in terms of equipment costs is the molten salt assisted synthesis of MAX phases [43–48]. This method showed sufficient efficiency in the preparation of individual MAX phases, e.g., Ti3SiC2, Ti2AlN, Ti3AlC2, and V2AlC, but it is rarely used to prepare more complex compositions in which one transition metal partially substitutes for another. Luo et al. [45], for example, described the synthesis of compounds in the (V1 – x – yTixCry)2AlC system in molten NaCl/KCl in the molar ratio 1 : 1 at 1100°С followed, after the salts were removed, by heat treatment under argon at 1400°С.
The preparation of MAX phases in the Ti2AlC–V2AlC system that would be interesting for the further synthesis of MXenes Ti2 – xVxCTy as components for energy and chemical gas sensors, is poorly documented [49–54]. For preparing a compound of composition (Ti0.5,V0.5)2AlC, for example, the precursor metal powders and graphite were encapsulated into borosilicate ampoules at 650°С and then heated to 1600°С under isostatic hot pressing conditions at 100 MPa for 8 h [49, 50]. The as-prepared (Ti0.5,V0.5)2AlC had a high oxidation resistance compared to V2AlC. In [51, 52], the synthesis of a compound of similar composition involved mixing TiH2, V, Al, and graphite powders in the molar ratios 1 : 1 : (0.9–1.2) : 1, stirring milling them in a ball mill with stearic acid, compaction into tablets, and in vacuo sintering under stepwise heating to 1500°С. Zou et al. [51] noted that the TiVAlC product contained metal carbides and metallic vanadium impurities. Yazdanparast et al. [53], in their preparation of heterometallic MXene TiVC, synthesized MAX phase TiVAlC as the precursor via hot pressing of powdered titanium, vanadium, aluminum, and graphite in the molar ratio 1 : 1 : 1.3 : 1 at 1450°С (the exposure time was 2 h). They observed a rather large amount of impurity Ti3AlC2, another MAX phase [53]. Wang et al. [54], in their study targeted at preparing MXenes (Vx,Ti1 – x)2C (х = 1, 0.7, 0.5, 0.3, 0), prepared the relevant aluminum-containing MAX phases via milling of titanium, vanadium, aluminum, and graphite powders (n(Ti + V) : n(Al) : n(C) = 2 : 1.3 : 1) and heat treatment at 1450°С for 2 h. Judging from X-ray diffraction patterns [54], those solid solutions featured significant amounts of MAX phase M3AlC2.
The purpose of this paper is to study the possibility of a relatively low-temperature molten salt assisted synthesis to provide MAX phases in the Ti2AlC–V2AlC system containing the lowest possible amounts of impurities that would be relatively easy to remove in the preparation of corresponding MXenes.
EXPERIMENTAL
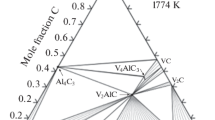
The reagents used, namely powders of metallic titanium (99.9%, 0.5–100 µm), aluminum (99.2%, 30 µm), vanadium (99.9%, 40–100 µm), graphite (type MPG-8), potassium bromide (99%, Ruskhim), were mixed in molar ratios n(Ti + V) : n(Al) : n(C) = 2 : 1.2 : 0.8. These molar ratios slightly differ from the rations n(Ti + V) : n(Al) : n(C) = 2 : 1.1 : 0.9–1 proposed in most works, due to our decision to neglect the loss of desired phases in favor of producing impurities that would be relatively easy to remove during the synthesis of MXenes (excessive precursor metals, titanium aluminides, vanadium, and possibly aluminum oxide). The titanium and vanadium ratios were chosen to satisfy the following formulas: Ti2AlC, Ti1.8V0.2AlC, Ti1.5V0.5AlC, Ti1V1AlC, Ti0.5V1.5AlC, Ti0.2V1.8AlC, and V2AlC; that is, vanadium should substitute for part of titanium atoms in the course of MAX phase synthesis (Fig. 1). Next, dried potassium bromide powder was added to the mixed powder in the weight ratio 1 : 1 [43, 55]. The mixture was homogenized in a PM 100 (Retsch) planetary ball mill using a ZrO2 milling drum and WC balls in ethanol at 300 rpm for 12 h. After the milled product was dried at 70–80°С, tablets 2 cm in diameter and 1–1.5 cm thick were formed, and then were placed into Alundum crucibles and covered with the KBr powder. MAX phase synthesis was fulfilled in a muffle at 1000°С for 5 h (the first series) and at 1100°С for 3 h (the second series). Once cooled fully, the samples were washed with hot water and then dried at 110–120°С.
The X-ray diffraction patterns of MAX phase samples were recorded on a Bruker D8 Advance diffractometer (CuKα radiation, 0.02° resolution with a signal accumulation time of 0.3 s per point). Phase identification was fulfilled in the software MATCH!— Phase Identification from Powder Diffraction, Version 3.8.0.137 (Crystal Impact, Germany) using the Crystallography Open Database (COD) supplemented with data on Ti2AlC and V2AlC hexagonal phases.
The thermal behavior of the prepared MAX phase powders in flowing air was studied on an SDT Q-600 TG/DSC/DTA simultaneous thermal analyzer in the range 20–1000°С; the heating rate was 10 deg/min, the air flow rate was 250 mL/min.
The microstructure of the prepared Ti2AlC and V2AlC phases was studied by transmission electron microscopy (TEM, Jeol JEM-1011 equipped with an Orius SC1000W digital camera). For Ti2AlC, the particle morphology was in addition studied by scanning electron microscopy (SEM) on an NVision 40 (Carl Zeiss) three-beam workstation; the elemental composition was estimated in 45 × 60 µm areas by means of an EDX Oxford Instruments X-MAX 80 attachment.
RESULTS AND DISCUSSION
The phase identification of the products synthesized at 1000°С (Fig. 2) showed that a MAX phase of 211 stoichiometry (M2AlC) was formed in all compositions, as evidenced by the characteristic set of reflections, especially the strong (002) reflection with its intrinsic systematic shift upon partial substitution of vanadium for titanium. Minor MAX phase M3AlC2 was not formed under the conditions used. The Ti2AlC [1, 56] and V2AlC [57] individual phases exceeded 82 and 86% respectively. The major impurities were aluminum oxide [58] (8–10%), titanium carbide [59] and vanadium carbide [60] (4 and 1.6%, respectively), Ti3Al [61], TiAl, and metallic vanadium [62]. The situation with intermediate phases is significantly different, especially for Ti1V1AlC, Ti0.5V1.5AlC, and Ti0.2V1.8AlC. Very strong partially overlapping reflections from Al2O3 and vanadiuum impurity phases appear near the strongest reflection of the mixed MAX phase.
The full-profile analysis in the TOPAS software gave average unit cell parameters (Fig. 3, blue curve). From Fig. 3, one can see the following: while parameter c only slight deviates from Vegrad’s law (which may indicate increased spacings between carbide planes in the MAX phase lattice), the parameter a = 3.11 Å for composition Ti1V1AlC even exceeds the respective value for individual Ti2AlC (a = 3.04–3.06 Å [1, 56]). Not only this observation signifies that some vanadium does not enter the strucure of the newly formed MAX phase, but it can also signify a significant disorder in M2C planes.
Since the formation of a single-phase Ti1V1AlC with unit cell parameters close to the calculated values is documented in the literature, proceeding from the additive scheme (a = 2.98 Å, с = 13.42 Å [50]) we decided to carry out an additional experiment to synthesize Ti1V1AlC, Ti0.5V1.5AlC, and Ti0.2V1.8AlC at 1100°С.
The 100°С increase in synthesis temperature led to a significant decrease in mounts of Al2O3 and excessive vanadium in the samples (Fig. 4). Although the Ti0.5V1.5AlC sample had the least phase purity as judged from its X-ray diffraction patterns, the unit cell parameters calculated by full-profile analysis (Fig. 3, pink curve) showed that Ti1V1AlC yet featured the greatest deviation from the linearity of the unit cell parameter a = 3.07 Å depending MAX phase composition. However, there is an obvious tendency for the unit cell parameters to approach the theoretical values as the synthesis temperature increases.
The data imply that the significantly larger values of the unit cell parameter a, typical of the Ti1V1AlC samples prepared either at 1000°С or at 1100°С (with its actual coincidence with the calculated parameter c), are probably associated with disorder in M2C layers. One more argument in favor of this conclusion comes from the theoretical work [63], in which the Ti2AlC–V2AlC system was classified with the group of systems for which the disorder-to-order transition requires an elevated temperature (~1500°C). It is also worth mentioning that the preparation of solid solutions of the above system at 1450–1600°С is documented in the available literature. In this work, we found that the molten salt assisted synthesis does produce MAX phase solid solutions in this system (except for Ti1V1AlC) even at as low temperature as 1000–1100°С.
The oxidation resistance in flowing air was studied for the end-members (MAX phases Ti2AlC and V2AlC) and for Ti1V1AlC samples (Fig. 5). In every sample, temperature elevation gives rise exclusively to a weight gain owing to the oxidation of the produced hexagonal carbides and some impurities in them. The oxidative resistance of Ti2AlC far exceeds that for V2AlC: the observable weight increment for Ti2AlC starts at ~480°С against ~400°С for V2AlC (Figs. 5a, 5b). In addition, oxidation in the Ti2AlC sample is a stepped process, and its greatest development is observed at temperatures above 800°С (with attendant exotehrms peaking at 821 and 907°С). For V2AlC, two well-defined oxidation steps can likewise be recognized but at far lower temperatures: in the range 500–600°С and at 600–650°С. Noteworthy, the V2AlC sample does not experience weight gain at 700°C.
For the Ti1V1AlC samples prepared at 1000 or 1100°С (Figs. 5c and 5d, respectively), it is difficult to recognize pronounced oxidation steps on DSC and TG curves. In either sample, weight gain starts in the range 440–450°С, which is an intermediate value between those in the Ti2AlC and V2AlC samples. A sharp weight gain occurs in the range 500–700°С, with an attendant exotherm peaking at 593°С (for the sample prepared at 1000°С). For the Ti1V1AlC sample syntehsized at 1100°С, the exotherm peak temperature shifts toward lower temperatures to 584°С, but a shoulder appears at 616°С. Thus, we may state that the oxidation resistance systematically decreases as vanadium-for-titanium substitution progresses in the Ti2AlC phase.
The TEM microstructure study of V2AlC powder (Fig. 6) showed that the powder was comprised of strong intergrowths of particles (400 nm or more in diameter), often with hexagonal faceting. Since the compound tends to cleavage owing to its structure, areas of different thickness appear on the micrographs, and Figs. 6e and 6f feature a stratified chip of a particle on which steps of planes are clearly visible. In addition, arrows show the areas of the particles with the moire pattern, which is typical of layered structures.
Similar inferences may refer to the Ti2AlC sample as well (Fig. 7). On can see on its TEM micrographs that the powder is comprised of intergrown aggregates of particles with sizes of 200–300 nm or more; the moire pattern on them indicates their layered structure. In addition, Figs. 7e and 7f feature a set of exfoliated flat particles, also covered with a moire pattern.
The SEM images (Fig. 8) also verify that the Ti2AlC sample is comprised of layered particles. Figure 8a shows a chip of such an entity and crack propagation with deviations on some layers typical of MAX phases. Imaging in the average atomic number contrast mode (Fig. 8b) indicates the absence of large impurity particles in the product; they can predominantly be distributed along grain boundaries and in-between grains. Energy dispersive analysis shows that the molar ratio n(Ti) : n(С) is nearly as specified. The aluminum amount slightly exceeds the Ti2AlC stoichiometry but is lower than entered the reaction, likely due to loss in the course of synthesis, specifically due to oxidation upon heating before the molten potassium bromide shielding layer was formed.
CONCLUSIONS
We have considered the details of relatively low-temperature molten salt assisted synthesis (at 1000 and 1100°С) of MAX phase solid solutions in the Ti2AlC–V2AlC system.
The developed method provides desired phases whose exceeds 82–86% in the product, and avoids formation of large amounts of impurities that are difficult to remove during subsequent MXene preparation, primarily highly disperse titanium and vanadium monocarbides (their percentage is within 1.6–4%). In addition, neither of the prepared samples featured impurities of other MAX phases, e.g., M3AlC2, while their presence in complex MAX phases in the system of interest was documented elsewhere.
When the synthesis temperature is 1000°С, Ti1V1AlC, Ti0.5V1.5AlC, and Ti0.2V1.8AlC samples (despite the formation of a MAX phase and a systematic shift of reflection (002) in response to progressing of vanadium-for-titanium substitution) feature appreciable amounts of Al2O3 and vanadium (their reflections are partially overlapping). Full-profile analysis shows that the unit cell parameter a in a Ti1V1AlC sample is even superior to the respective value in individual Ti2AlC. An increase in synthetic temperature to 1100°С appreciably reduces the amount of impurities; the unit cell parameter a also decreases, but yet appreciably exceeds the calculated and published values [50] (while the parameter c corresponds with those values). Likely, this situation is not due to the ratio n(Ti) : n(V) in a conventional M2C plane, but due to disorder, and higher heat-treatment temperatures can help to level it out [63].
Thermal analysis of Ti2AlC, Ti1V1AlC, and V2AlC samples in the range 20–1000°С shows that the oxidation resistance decreases as vanadium-for-titanium substitution in a MAX phase progresses.
The microstructures of prepared Ti2AlC and V2AlC phases have been studied by TEM and SEM.
REFERENCES
W. Jeitschko, H. Nowotny, and F. Benesovsky, Monatsh. Chem. 94, 672 (1963). https://doi.org/10.1007/BF00913068
W. Jeitschko, H. Nowotny, and F. Benesovsky, Monatsh. Chem. 95, 431 (1964). https://doi.org/10.1007/BF00901306
W. Jeitschko, H. Nowotny, and F. Benesovsky, Monatsh. Chem. 94, 1198 (1963). https://doi.org/10.1007/BF00905710
W. Jeitschko, H. Nowotny, and F. Benesovsky, Monatsh. Chem. 95, 156 (1964). https://doi.org/10.1007/BF00909261
H. Nowotny, W. Jeitschko, and F. Benesovsky, Planseeber. Pulvermetall. 12, 31 (1964).
A. Zhou, Y. Liu, S. Li, et al., J. Adv. Ceram. 10, 1194 (2021). https://doi.org/10.1007/s40145-021-0535-5
N. F. Rosli, M. Z. M. Nasir, N. Antonatos, et al., ACS Appl. Nano Mater. 2, 6010 (2019). https://doi.org/10.1021/acsanm.9b01526
J. Gonzalez-Julian, J. Am. Ceram. Soc. 104, 659 (2021). https://doi.org/10.1111/jace.17544
J. Lyu, E. B. Kashkarov, N. Travitzky, et al., J. Mater. Sci. 56, 1980 (2021). https://doi.org/10.1007/s10853-020-05359-y
X. Lei and N. Lin, Crit. Rev. Solid State Mater. Sci., 1 (2021). https://doi.org/10.1080/10408436.2021.1966384
L. Fu and W. Xia, Adv. Eng. Mater. 23, 2001191 (2021). https://doi.org/10.1002/adem.202001191
A. Dash, J. Malzbender, M. Rasinski, et al., Materialia 21, 101295 (2022). https://doi.org/10.1016/j.mtla.2021.101295
G. Mayer, Am. Ceram. Soc. Bull. 83, E5/1 (2004).
M. M. Opeka, I. G. Talmy, and J. A. Zaykoski, J. Mater. Sci. 39, 5887 (2004). https://doi.org/10.1023/B:JMSC.0000041686.21788.77
K. A. Kane, B. A. Pint, D. Mitchell, et al., J. Eur. Ceram. Soc. 41, 6130 (2021). https://doi.org/10.1016/j.jeurceramsoc.2021.05.055
Q. Guo, C. Juvencio Da Silva, B. Bourgeois, et al., J. Ceram. Soc. Jpn. 126, 16 (2018). https://doi.org/10.2109/jcersj2.17027
Q. Guo, C. V. J. da Silva, B. B. Bourgeois, et al., Ceram. Int. 43, 13047 (2017). https://doi.org/10.1016/j.ceramint.2017.05.149
L. Silvestroni, C. Melandri, and J. Gonzalez-Julian, J. Eur. Ceram. Soc. 41, 6064 (2021). https://doi.org/10.1016/j.jeurceramsoc.2021.05.029
M. Shahedi Asl, B. Nayebi, M. Akhlaghi, et al., Ceram. Int. 47, 817 (2021). https://doi.org/10.1016/j.ceramint.2020.08.193
E. P. Simonenko, N. P. Simonenko, A. N. Gordeev, et al., J. Eur. Ceram. Soc. 41, 1088 (2021). https://doi.org/10.1016/j.jeurceramsoc.2020.10.001
E. P. Simonenko, N. P. Simonenko, A. F. Kolesnikov, et al., J. Eur. Ceram. Soc. 42, 30 (2022). https://doi.org/10.1016/j.jeurceramsoc.2021.09.020
E. P. Simonenko, N. P. Simonenko, A. F. Kolesnikov, et al., Russ. J. Inorg. Chem. 66, 1405 (2021). https://doi.org/10.1134/S003602362109014X
J. W. Kemp, A. A. Diaz, E. C. Malek, et al., Addit. Manuf. 44, 102049 (2021). https://doi.org/10.1016/j.addma.2021.102049
S. K. Thimmappa, B. R. Golla, S. B. Pitchuka, et al., Ceram. Int. 47, 22184 (2021). https://doi.org/10.1016/j.ceramint.2021.04.241
E. P. Simonenko, N. P. Simonenko, A. N. Gordeev, et al., Russ. J. Inorg. Chem. 65, 1596 (2020). https://doi.org/10.1134/S0036023620100198
X. Huo, J. Zhong, Z. Yang, et al., ACS Appl. Mater. Interfaces 13, 55112 (2021). https://doi.org/10.1021/acsami.1c16706
B. Lu, Z. Zhu, B. Ma, et al., Small. 17, 2100946 (2021). https://doi.org/10.1002/smll.202100946
S.-S. Li, X.-H. Cui, X.-H. Li, et al., Vacuum. 196, 110774 (2022). https://doi.org/10.1016/j.vacuum.2021.110774
M. Zhang, Y. Yang, Y. Wang, et al., Food Control 132, 108532 (2022). https://doi.org/10.1016/j.foodcont.2021.108532
Q. Xu, B. Zong, Q. Li, et al., J. Hazard. Mater. 424, 127492 (2022). https://doi.org/10.1016/j.jhazmat.2021.127492
H. Pazniak, I. A. Plugin, M. J. Loes, et al., ACS Appl. Nano Mater. 3, 3195 (2020). https://doi.org/10.1021/acsanm.9b02223
H. Pazniak, A. S. Varezhnikov, D. A. Kolosov, et al., Adv. Mater. 33, 2104878 (2021). https://doi.org/10.1002/adma.202104878
J. Michael, Z. Qifeng, and W. Danling, Nanomater. Nanotechnol. 9, (2019). https://doi.org/10.1177/1847980418824470
X. Zheng, J. Alloys Compd. 899, 163275 (2022). https://doi.org/10.1016/j.jallcom.2021.163275
C. Liu, J. Zhou, X. Li, et al., Chem. Eng. J. 431, 133838 (2022). https://doi.org/10.1016/j.cej.2021.133838
T. Najam, S. S. A. Shah, L. Peng, et al., Coord. Chem. Rev. 454, 214339 (2022). https://doi.org/10.1016/j.ccr.2021.214339
Y. Cao, Y. Guo, Z. Chen, et al., Nano Energy 92, 106689 (2022). https://doi.org/10.1016/j.nanoen.2021.106689
G. Li, X. Zhong, X. Wang, et al., Bioact. Mater. 8, 409 (2022). https://doi.org/10.1016/j.bioactmat.2021.06.021
Q. He, H. Hu, J. Han, et al., Mater. Lett. 308, 131100 (2022). https://doi.org/10.1016/j.matlet.2021.131100
W. Bao, X.-G. Wang, H. Ding, et al., Scr. Mater. 183, 33 (2020). https://doi.org/10.1016/j.scriptamat.2020.03.015
X. H. Chen, H. X. Zhai, S. B. Li, et al., Adv. Mater. Res. 105-106, 83 (2010). https://doi.org/10.4028/www.scientific.net/AMR.105-106.83
D. Bowden, J. Ward, S. Middleburgh, et al., Acta Mater. 183, 24 (2020). https://doi.org/10.1016/j.actamat.2019.10.049
A. Dash, R. Vaßen, O. Guillon, et al., Nat. Mater. 18, 465 (2019). https://doi.org/10.1038/s41563-019-0328-1
A. Liu, Q. Yang, X. Ren, et al., Ceram. Int. 46, 6934 (2020). https://doi.org/10.1016/j.ceramint.2019.11.008
W. Luo, Y. Liu, C. Wang, et al., J. Mater. Chem. 9, 7697 (2021). https://doi.org/10.1039/D1TC01338F
C. Roy, P. Banerjee, and S. Bhattacharyya, J. Eur. Ceram. Soc. 40, 923 (2020). https://doi.org/10.1016/j.jeurceramsoc.2019.10.020
B. Wang, A. Zhou, Q. Hu, et al., Int. J. Appl. Ceram. Technol. 14, 873 (2017). https://doi.org/10.1111/ijac.12723
S. Badie, A. Dash, Y. J. Sohn, et al., J. Am. Ceram. Soc. 104, 1669 (2021). https://doi.org/10.1111/jace.17582
S. Gupta and M. W. Barsoum, J. Electrochem. Soc. 151, D24 (2004).
B. Manoun, F. Zhang, S. K. Saxena, et al., J. Phys.: Condens. Matter. 19, 246215 (2007). https://doi.org/10.1088/0953-8984/19/24/246215
H. Zou, X. Li, C. Zhang, et al., Ceram. Int. 47, 8338 (2021). https://doi.org/10.1016/j.ceramint.2020.11.196
H. Zou, X. Li, C. Zhang, et al., Ceram. Int. 47, 28288 (2021). https://doi.org/10.1016/j.ceramint.2021.06.245
S. Yazdanparast, S. Soltanmohammad, A. Fash-White, et al., ACS Appl. Mater. Interfaces 12, 20129 (2020). https://doi.org/10.1021/acsami.0c03181
Y. Wang, W. Zheng, P. Zhang, et al., J. Mater. Sci. 54, 11991 (2019). https://doi.org/10.1007/s10853-019-03756-6
P. Bärmann, L. Haneke, J. M. Wrogemann, et al., ACS Appl. Mater. Interfaces 13, 26074 (2021). https://doi.org/10.1021/acsami.1c05889
S. Sridharan, H. Nowotny, and S. F. Wayne, Monatshefte fur Chemie Chem. Mon. 114, 127 (1983). https://doi.org/10.1007/BF00798317
J. C. Schuster and H. Nowotny, Z. Metallkd. 71, 341 (1980). http://inis.iaea.org/search/search.aspx?orig_q = RN:11558060
E. Herlinger, Angew. Chem. 42, 797 (1929). https://doi.org/10.1002/ange.19290423015
R. W. G. Wyckoff, Structure of Crystals, Second Ed. (The Chemical Catalog Company, INC, New York, 1931).
R. W. G. Wyckoff, Cryst. Struct. 1, 85 (1963).
T. Novoselova, S. Malinov, W. Sha, et al., Mater. Sci. Eng., A 371, 103 (2004). https://doi.org/10.1016/j.msea.2003.12.015
A. W. Hull, Phys. Rev. 20, 113 (1922).
M. Dahlqvist and J. Rosen, Phys. Chem. Chem. Phys. 17, 31810 (2015). https://doi.org/10.1039/C5CP06021D
Funding
The work was supported by the Russian Science Foundation (Project No. 21-73-10251), https://rscf.ru/project/21-73-10251/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simonenko, E.P., Simonenko, N.P., Nagornov, I.A. et al. Synthesis of MAX Phases in the Ti2AlC–V2AlC System as Precursors of Heterometallic MXenes Ti2 – xVxC. Russ. J. Inorg. Chem. 67, 705–714 (2022). https://doi.org/10.1134/S0036023622050187
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050187