Abstract
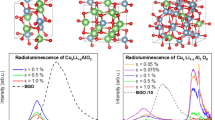
Ceramic lithium aluminum spinels doped with manganese ions have been synthesized by a high-temperature solid-state reaction. Samples fabricated by annealing at 1000, 1100, 1200, or 1300°C in air for 4 h have been identified as LiAl5O8, which is cubic spinel belonging to the space group P4132. After annealing at 1000–1200°C, samples with the lattice parameter a = 7.923–7.925 Å show intense red luminescence of Mn4+ ions, which has a narrow luminescence band with a maximum at 662 nm. The excess charge of Mn4+ ions replacing Al3+ ions in the octahedral sites of the LiAl5O8 lattice is compensated for by an excess amount of lithium ions (compared to the stoichiometry) substituting for Al3+ ions adjacent to Mn4+. However, annealing at a higher temperature (1300°C) leads to the almost complete disappearance of the Mn4+ luminescence at 662 nm, although X-ray powder diffraction shows that the crystal structure of these ceramics remains the same one (space group P4132), but the lattice parameter a becomes 7.908 Å, which exactly corresponds to the lattice parameter of the LiAl5O8 single crystal. It is suggested that ceramics with a larger lattice parameter obtained at lower temperatures are solid solutions in which some of the Al3+ ions are replaced by larger Li+ ions. Such solid solutions are not stable and lose Li+ ions upon annealing at a high temperature to convert to the stoichiometric compound LiAl5O8. In stoichiometric LiAl5O8, Mn4+ ions cannot be stabilized in the octahedral sites due to the lack of a charge compensation mechanism, and there is no red luminescence of Mn4+ ions in such samples.









Similar content being viewed by others
REFERENCES
G. B. Nair, H. C. Swart, and S. J. Dhoble, Prog. Mater. Sci 109, 100622 (2020). https://doi.org/10.1016/j.pmatsci.2019.100622
Q. Zhou, L. Dolgov, A. M. Srivastava, et al., J. Mater. Chem. C 6, 2652 (2018). https://doi.org/10.1039/c8tc00251g
S. Adachi, J. Lumin. 202, 263 (2018). https://doi.org/10.1016/j.jlumin.2018.05.05
S. Adachi, ECS J. Solid State Sci. Technol. 9, 016001 (2020). https://doi.org/10.1149/2.0022001JSS
Y. H. Kim, J. Ha, and W. B. Im, J. Mater. Res. Technol. 11, 181 (2021). https://doi.org/10.1016/j.jmrt.2021.01.011
S. J. Dhoble, R. Priya, N. S. Dhoble, and O. P. Pandey, Luminescence 36, 560 (2021). https://doi.org/10.1002/bio.3991
L. Meng, L. Liang, and Y. Wen, Sci. Adv. Mater. 9, 456 (2017). https://doi.org/10.1166/sam.2017.2320
T. Jansen, J. Gorobez, M. Kirm, et al., ECS J. Solid State Sci. Technol. 7, R3086 (2018). https://doi.org/10.1149/2.0121801jss
A. J. S. Silva, S. M. Freitas, P. A. Nascimento, et al., Ceram. Int. 45, 18994 (2019). https://doi.org/10.1016/j.ceramint.2019.06.140
V. C. Teixeira, A. J. S. Silva, I. F. Manali, et al., Opt. Mater. 94, 160 (2019). https://doi.org/10.1016/j.optmat.2019.05.029
R. Famery, F. Queyroux, J.-C. Gilles, et al., J. Solid State Chem. 30, 257 (1979). https://doi.org/10.1016/0022-4596(79)90107-5
M. Kriens, G. Adiwidjaja, W. Guse, et al., Neues Jahrb. Mineral. Monatsh. 8, 344 (1996).
K. Momma and F. Izumi, J. Appl. Crystallogr. 44, 1272 (2011). https://doi.org/10.1107/S0021889811038970
A. Jain, S. P. Ong, G. Hautier, et al., APL Mater. 1, 011002 (2013). https://doi.org/10.1063/1.4812323
B. D. McNicol and G. T. Pott, J. Lumin. 6, 320 (1973). https://doi.org/10.1016/0022-2313(73)90027-6
N. M. Khaidukov, M. N. Brekhovskikh, N. Yu. Kirikova, et al., Ceram. Int. 46, 21351 (2020). https://doi.org/10.1016/j.ceramint.2020.05.231
N. M. Khaidukov, M. N. Brekhovskikh, N. Yu. Kirikova, et al., Russ. J. Inorg. Chem. 65, 1135 (2020). https://doi.org/10.1134/S0036023620080069
S. Kh. Batygov, M. N. Brekhovskikh, L. V. Moiseeva, et al., Russ. J. Inorg. Chem. 66, 1491 (2021). https://doi.org/10.1134/S0036023621100028
M. Aoyama, Y. Amano, K. Inoue, et al., J. Lumin. 136, 411 (2013). https://doi.org/10.1016/j.jlumin.2012.12.012
R. D. Shannon, Acta Crystallogr., Sect. A 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
Y. Tanabe and S. Sugano, J. Phys. Soc. Jpn. 9, 776 (1954). https://doi.org/10.1143/JPSJ.9.766
H. Ji, X. Hou, M. Molokeev, et al., Dalton Trans. 49, 5711 (2020). https://doi.org/10.1039/D0DT00931H
S. P. Feofilov, A. B. Kulinkin, and N. M. Khaidukov, J. Lumin. 217, 116824 (2020). https://doi.org/10.1016/j.jlumin.2019.116824
G. T. Pott and B. D. McNicol, J. Solid State Chem. 7, 132 (1973). https://doi.org/10.1016/0022-4596(73)90145-X
ACKNOWLEDGMENTS
The studies were performed by using equipment of the Joint Research Center at the Kurnakov Institute of General and Inorganic Chemistry, RAS, and the Lebedev Physical Institute, RAS.
Funding
The study was supported by the Russian Science Foundation (RSF) (grant no.18-13-00407).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Khaidukov, N.M., Brekhovskikh, M.N., Kirikova, N.Y. et al. Specific Features of Synthesis and Luminescence for Lithium Aluminum Spinel LiAl5O8 Doped with Manganese Ions. Russ. J. Inorg. Chem. 67, 547–554 (2022). https://doi.org/10.1134/S003602362204009X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362204009X