Abstract
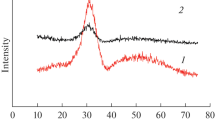
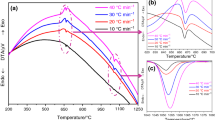
Ln2CrTaO7 (Ln = Sm, Gd, Y) pyrochlores are synthesized by the co-precipitation with subsequent annealing. The effect of the precursor composition ((NH4)2Cr2O7, Cr(NO3)3, and CrCl3) on the precipitates reactivity and product microstructure was studied. Samples with an average particle size of 200 nm for Y2CrTaO7, 450 nm for Gd2CrTaO7 and 600 nm for Sm2CrTaO7 were used to study thermodynamic properties. The temperature dependences of heat capacity were measured by the adiabatic calorimetry (13–346.16 K) method and the ratio method using DSC measurements (330–1300 K). The thermodynamic functions of Ln2CrTaO7 compounds were calculated. The absence of polymorphic transitions up to a temperature of 1450°C for all studied compounds is shown.





Similar content being viewed by others
REFERENCES
C. K. Hao, H. E. Hung, and C. S. Lee, Sol-Gel Sci. Technol. 76, 428 (2015). https://doi.org/10.1007/s10971-015-3791-4
B. J. Wuensch, K. W. Eberman, C. Heremans et al., Solid State Ion. 129, 111 (2000). https://doi.org/10.1016/S0167-2738(99)00320-3
M. Sun and B. Huang, Inorg. Chem. 56, 7975 (2017). https://doi.org/10.1021/acs.inorgchem.7b00683
S. H. Oh, R. Black, E. Pomerantseva, et al., Nat. Chem. 4, 1004 (2012). https://doi.org/10.1038/nchem.1499
Y. Liu, R. L. Withers, H. Chen, et al., Curr. Appl. Phys. 11, 171 (2011). https://doi.org/10.1016/j.cap.2011.03.014
J. P. Luan, X. P. Hao, S. R. Zheng, et al., J. Mater. Sci. 41, 8001 (2006). https://doi.org/10.1007/s10853-006-0869-y
Z. Zou, J. Ye, and H. Arakawa, Int. J. Hydrogen Energy 28, 663 (2003). https://doi.org/10.1016/S0360-3199(02)00159-3
K. L. Rosas-Barrera, J. L. Ropero-Vega, J. A. Pedraza-Avella, et al., Catal. Today. 166, 135 (2011). https://doi.org/10.1016/j.cattod.2010.08.008
O. G. Ellert, A. V. Egorysheva, E. Y. Liberman, et al., Inorg. Mater. 55, 1257 (2019). https://doi.org/10.1134/S0020168519120033
Z. G. Lu, J. W. Wang, Y. G. Tang, et al., J. Solid State Chem. 177, 3075 (2004). https://doi.org/10.1016/j.jssc.2004.04.053
S. K. Gupta, J. P. Zuniga, M. Abdou, et al., Inorg. Chem. Front. 7, 505 (2020). https://doi.org/10.1039/C9QI01181A
R. S. Pavlov, J. B. C. Castello, V. B. Marza, et al., J. Am. Ceram. Soc. 85, 1197 (2002). https://doi.org/10.1039/B201802K
V. D. Risovany, A. V. Zakharov, E. M. Muraleva, et al., J. Nucl. Mater. 355, 163 (2006). https://doi.org/10.1016/j.jnucmat.2006.05.029
R. C. Ewing and W. J. Weber, and J. Lian, J. Appl. Phys. 95, 5949 (2004). https://doi.org/10.1063/1.1707213
K. E. Sickafus, R. W. Grimes, J. A. Valdez, et al., Nature Mater. 6, 216 (2007). https://doi.org/10.1038/nmat1842
J. S. Gardner, M. J. P. Gingras, and J. E. Greedan, Rev. Mod. Phys. 82, 53 (2010). https://doi.org/10.1103/revmodphys.82.53
A. V. Egorysheva, O. G. Ellert, D. I. Kirdyankin, et al., J. Magn. Magn. Mater. 513, 167226 (2020). https://doi.org/10.1016/j.jmmm.2020.167226
X. Wan, A. M. Turner, A. Vishwanath, et al., Phys. Rev. B. 83, 205101 (2011). https://doi.org/10.1103/PhysRevB.83.205101
M. Ezawa, Phys. Rev. Lett. 120, 026801 (2018). https://doi.org/10.1103/PhysRevLett.120.026801
M. A. Subramanian, G. Aravamudan, and G. V. Subba Rao, Prog. Solid State Chem. 15, 55 (1983). https://doi.org/10.1016/0079-6786(83)90001-8
W. Pan, Q. Xu, L. H. Qi, et al., Key. Eng. Mater. 280, 1497 (2005). https://doi.org/10.4028/www.scientific.net/KEM.280-283.1497
N. P. Simonenko, K. A. Sakharov, E. P. Simonenko, et al., Russ. J. Inorg. Chem. 60, 1452 (2015). https://doi.org/10.1134/S0036023615120232
M. P. Schmitt, A. K. Rai, R. Bhattacharya, et al., Surf. Coat. Technol. 251, 56 (2014). https://doi.org/10.1016/j.surfcoat.2014.03.049
R. Vasser, X. Q. Cao, F. Tietz,et al., J. Am. Ceram. Soc. 83, 3031 (2000). https://doi.org/10.1111/j.1151-2916.2000.tb01506.x
J. Wu, X. Wei, N. P. Padture, et al., J. Am. Ceram. Soc. 85, 3031 (2002). https://doi.org/10.1111/j.1151-2916.2002.tb00574.x
J. Xiang, S. Chen, J. Huang, et al., Ceram. Intern. 38, 3607 (2012). https://doi.org/10.1016/j.ceramint.2011.12.077
L. Chen, M. Hu, P. Wu, et al., J. Am. Ceram. Soc. 102, 4809 (2019). https://doi.org/10.1111/jace.16328
M. Nyman, M. A. Rodriguez, and L. E. S. Rohwer, Chem. Mater. 21, 4731 (2009). https://doi.org/10.1021/cm9020645
S. Shu, Y. Wang, Y. Ke, et al., J. Alloys Compd. 848, 156359 (2020). https://doi.org/10.1016/j.jallcom.2020.156359
R. Dou, Q. Zhang, J. Gao, et al., Crystals 8, 55 (2018). https://doi.org/10.3390/cryst8020055
L. Chen, Y. Jiang, X. Chong, et al., J. Am. Ceram. Soc. 101, 1266 (2018). https://doi.org/10.1111/jace.15268
J. Wang, X. Chong, R. Zhou, et al., Scripta Mater. 126, 24 (2017). https://doi.org/10.1016/j.scriptamat.2016.08.019
Y. Haipeng, C. Xiaoge, Z. Hongsong, et al., Cogent Physics 3, 1244244 (2016). https://doi.org/10.1080/23311940.2016.1244244
T. An, L. Guofang, C. Zhen, et al., Ceram. Int. 44, 19160 (2018). https://doi.org/10.1016/j.ceramint.2018.06.229
J. Yang, Y. Han, M. Shahid, et al., Scripta Mater. 149, 49 (2018). https://doi.org/10.1016/j.scriptamat.2018.02.005
Q. Zheng, L. Chen, P. Song, et al., J. Alloys Comp. 855 157408 (2021). https://doi.org/10.1016/j.jallcom.2020.157408
A. V. Egorysheva, E. F. Popova, A. V. Tyurin, et al., Russ. J. Inorg. Chem. 64, 1342 (2019). https://doi.org/10.1134/S0036023619110056
M. E. Wieser, Pure Appl. Chem. 78, 2051 (2006). https://doi.org/10.1351/pac2006781112051
ACKNOWLEDGMENTS
The investigations were carried out using the facilities of the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Funding
This study was financially supported by the Russian Science Foundation (project no. 18-13-00025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Egorysheva, A.V., Popova, E.F., Tyurin, A.V. et al. Synthesis and Thermodynamic Properties of the Ln2CrTaO7 (Ln = Sm, Gd, Y) Pyrochlores. Russ. J. Inorg. Chem. 66, 1649–1659 (2021). https://doi.org/10.1134/S003602362111005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362111005X