Abstract
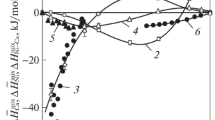
The partial and integral thermodynamic functions of mixing and evaporation for the melts of Pb–Ca system have been calculated from the vapor pressure data determined by boiling point method with involvement of published data. The formation of alloys containing <66.67 at % Ca is endothermic and accompanied by disorder growth, but it is exothermic at >66.67 at % Ca and leads to ordering in the system. Melting enthalpy of Ca2Pb of 13 ± 1.6 kJ/mol was found from the difference in sublimation and evaporation enthalpies. Based on the data on vapor pressure for the components, Pb–Ca constitution diagram has been supplemented by the fields of liquid and vapor coexistence under atmospheric pressure and vacuum of 1.33 and 0.7 kPa. The existence of two azeotropic mixtures with calcium concentration of 37.6 and 81.3 at % and boiling points of 2217 and 1635°C (2490 and 1908 K), respectively, has been revealed. When pressure (boiling point) decreases, the composition of azeotropic mixtures change according to Vrevskii laws. The position of field borders of vapor–liquid equilibrium indicates the impossibility of separation of Pb–Ca system into metals by distillation.





Similar content being viewed by others
REFERENCES
G. S. Rozhavskii and M. P. Smirnov, Tsvetn. Met. (Moscow, Russ. Fed.), No. 6, 13 (1961).
P. I. Fedorov and V. I. Shachnev, Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 3, 77, (1963).
M. P. Smirnov, Lead Refining and Intermediate Products Processing (Metallurgiya, Moscow, 1977) [in Russian].
A. M. Kunaev, S. M. Kozhakhmetov, A. V. Vanyukov, et al., Principles of Integrated Use of Feedstock in Non-Ferrous Metallurgy: Theory, Technology, and Implementation of New Metallurgical Processes (Nauka, Alma-Ata, 1982) [in Russian].
V. N. Volodin and R. A. Isakova, Distillation Processes for Separation of Sulfide and Metallic Melts: Theory and Technology (Tengri Ltd., Karaganda, 2015) [in Russian]. https://doi.org/10.31643/2015-2019.003
S. A. Shchukarev, M. P. Morozova, and G. F. Pron’, Zh. Obshch. Khim. 32, 2069 (1962).
V. G. Muradov and P. V. Gel’d, Uchenye zapiski Ul’yanovskogo gos. pedagogicheskogo in-ta. 20 (4), 116 (1966).
V. G. Muradov and V. Ya. Gabeskiriya, Uchenye Zapiski Ul’yanovskogo Gos. Pedagogicheskogo In-ta 21 (9), 3 (1966).
V. G. Muradov, P. V. Gel’d, and P. V. Kocherov, Zh. Fiz. Khim. 41, 576 (1967).
R. Hultgren, P. D. Desai, and D. T. Hawkins, et al., Selected Values of the Thermodynamic Properties of Binary Alloys (New York, 1973).
M. Notin, L. Bouirden, E. Belbacha, and J. Hertz, J. Less-Common Met. 154, 121 (1989).
F. Sommer, G. Borzone, N. Parodi, and R. Ferro, Intermetallics 14, 287 (2006).
B. P. Burylev, Thermodynamic and Thermochemical Constants (Nauka, Moscow, 1970) p. 32 [in Russian].
E. Schürmann and R. Schmid, Arch. Eisenhüttenwes. 46, 773 (1975).
V. M. Glazov, V. B. Lazarev, and V. V. Zharov, Phase Diagrams of Elementary Substances (Nauka, Moscow, 1980) [in Russian].
NianDai Yong and Yang Bing, Vacuum Metallurgy of Non-Ferrous Metals (Metallurgical Ind. Press, Beijing, 2000), Vol. 3.
V. P. Malyshev, A. M. Turdukozhaeva, E. A. Ospanov, and B. Sarkenov, Volatility and Boiling of Elementary Substances (Nauchnyi mir, Moscow, 2010) [in Russian].
Constitution Diagrams of Binary Metal Systems, Ed. by N. P. Lyakishev (Mashinostroenie, Moscow, 1996) [in Russian].
V. N. Volodin, V. E. Khrapunov, B. K. Kenzhaliev, et al., Izv. Vyssh. Uchebn. Zaved., Tsvetn. Metall., No. 3, 22 (2005).
Y. K. Rao, Metall. Trans. A 14, 308 (1983).
A. G. Morachevskii, Thermodynamics of Molten Metal and Salt Systems (Metallurgiya, Moscow, 1987) [in Russian].
V. N. Volodin, Liquid–Vapor Phase Transition in Binary Lead Systems under Low Pressure (Arko, Karaganda, 2012) [in Russian].
J. B. Clark and P. W. Richter, in Proceedings of the 7th AIRAPT Conference, Le Creusot,1979, High Pressure Science and Technology, Ed. by B. Vodar and Ph. Marteau (Pergamon, Oxford, UK, 1980), Vol. 1, p. 363.
M. S. Vrevskii, Solution Theory Researches (AN SSSR, Moscow, 1953) [in Russian].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Volodin, V.N., Tuleushev, Y.Z., Burabaeva, N.M. et al. Solution Thermodynamics and Aseotropic Mixtures in the Melts of Lead–Calcium System. Russ. J. Inorg. Chem. 65, 758–764 (2020). https://doi.org/10.1134/S0036023620050253
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620050253