Abstract
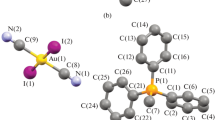
New ionic antimony complexes with a three-coordinated carbon atom in their cations, namely, [(Ph4SbO)3C]+[OSO2C6H3(NO2)2-2,4]– ⋅ 3PhH (32%) and [(p-Tol4SbO)3C]+Br– (traces), have been isolated when the products of reactions between pentaphenylantimony and 2,4-dinitrobenzenesulfonic acid and between penta(p-tolyl)antimony and hydrobromic acid were crystallized from a benzene–octane mixture in air along with the target products, such as tetraphenylantimony 2,4-dinitrobenzenesulfonate and tetra(p-tolyl)antimony bromide. The cations have nearly planar СO3Sb3 cores. The ОСО and COSb angles are close to 120°, whereas the C–O bonds vary within 1.277(4)–1.290(3) Å, and the Sb–O distances (2.266(2)–2.299(2) Å) exceed the sum of the covalent radii of antimony and oxygen atoms. It has been experimentally established that these complexes are formed as a result of interactions between the target products of the above-mentioned reactions with bis(tetraarylantimony) carbonates, which are also formed under the conditions of these reactions from pentaarylantimony and atmospheric carbon dioxide.





Similar content being viewed by others
REFERENCES
M. Hirai, M. Myahkostupov, F. N. Castellano, et al., Organometallics 35, 1854 (2016). https://doi.org/10.1021/acs.organomet.6b00233
D. Tofan and F. P. Gabbai, Chem. Sci. 7, 6768 (2016). https://doi.org/10.1039/C6SC02558G
S. Sen, I.-S. Ke, and F. P. Gabbai, Organometallics 36, 4224 (2017). https://doi.org/10.1021/acs.organomet.7b00654
L. Saleem, A. A. Altaf, and A. Badshah, et al., Inorg. Chim. Acta 474, 148 (2018). https://doi.org/10.1016/j.ica.2018.01.036
M. Hirai, J. Cho, and F. P. Gabbai, Chem.-Eur. J. 22, 6537 (2016). https://doi.org/10.1002/chem.201600971
S. Sen, I.-S. Ke, and F. P. Gabbai, Inorg. Chem. 55 (18), 9162 (2016). https://doi.org/10.1021/acs.inorgchem.6b01290
M. Yang and F. P. Gabbai, Inorg. Chem. 56, 8644 (2017). https://doi.org/10.1021/acs.inorgchem.7b00293
S. Sarwar, T. Iftikhar, and M. K. Rauf, Inorg. Chim. Acta 476, 12 (2018). https://doi.org/10.1016/j.ica.2018.02.005
K. A. Kocheshkov, A. P. Skoldinov, and N. N. Zemlyanskii, The Methods of Organoelement Chemistry. Antimony and Bismuth (Nauka, Moscow, 1976) [in Russian].
V. V. Sharutin and V. S. Senchurin, Named Reactions in the Chemistry of Organoelement Compounds (YuUrGU Press, Chelyabinsk, 2011) [in Russian].
G. K. Fukin, M. A. Samsonov, A. V. Arapova, et al., J. Solid State Chem. 254, 32 (2017). https://doi.org/10.1016/j.jssc.2017.06.030
A. I. Poddel’sky, M. V. Arsenyev, T. V. Astaf’eva, et al., J. Organomet. Chem. 835, 17 (2017). https://doi.org/10.1016/j.jorganchem.2017.02.035
T. Iftikhar, M. K. Rauf, S. Sarwar, et al., J. Organomet. Chem. 851, 89 (2017). https://doi.org/j.jorganchem.2017.09.002
A. I. Poddel’sky, I. V. Smolyaninov, G. K. Fukin, et al., J. Organomet. Chem. 824, 1 (2016). https://doi.org/10.1016/j.jorganchem.2016.09.021
A. I. Poddel’sky, I. V. Smolyaninov, G. K. Fukin, et al., J. Organomet. Chem. 867, 238 (2018). https://doi.org/10.1016/j.jorganchem.2017.12.006
C.-H. Chen and F. P. Gabbai, Angew. Chem., Int. Ed. Engl. 56, 1799 (2017). https://doi.org/10.1002/anie.201611009
A. M. Christianson and F. P. Gabbai, Chem. Commun. 53, 2471 (2017). https://doi.org/10.1039/C6CC09205E
X.-Y. Zhang, L. Cui, X. Zhang, et al., J. Mol. Struct. 1134, 742 (2017). https://doi.org/10.1016/j.molstruc.2017.01.039
SMART and SAINT-Plus: Data Collection and Processing Software for the SMART System, Versions 5.0 (Bruker, Madison, 1998).
SHELXTL/PC: An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Versions 5.10 (Bruker, Madison, 1998).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009). https://doi.org/10.1107/S0021889808042726
V. V. Sharutin, O. K. Sharutina, T. P. Platonova, et al., Russ. J. Gen. Chem. 71 (10), 1550 (2001).https://doi.org/10.1023/A:1013938600798
G. Ferguson and D. M. Hawley, Acta Crystallogr. 30, 103 (1974). https://doi.org/10.1107/S0567740874002299
V. V. Sharutin, O. K. Sharutina, L. P. Panova, and V. K. Bel’skii, Koord. Khim. 23, 513 (1997).
V. V. Sharutin, I. V. Egorova, A. P. Pakusina, et al., Russ. J. Coord. Chem. 33, 168 (2007).
G. Kolks, S. J. Lippard, and J. V. Waszczak, J. Am. Chem. Soc. 102, 4832 (1980). https://doi.org/10.1021/ja00534a045
A. Escuer, R. Vicente, E. Penalba, et al., Inorg. Chem. 35, 248 (1996). https://doi.org/10.1021/ic950331q
F. H. Allen, O. Kennard, D. G. Watson, et al., J. Chem. Soc., Perkin Trans. II (12), S1 (1987).
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation (grant no. 4.6151.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. & Efremov, A.N. Arylantimony Derivatives of Three-Coordinated Carbon. Russ. J. Inorg. Chem. 65, 45–51 (2020). https://doi.org/10.1134/S0036023620010155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010155