Abstract
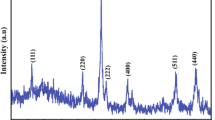
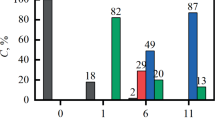
A method for the preparation of high-purity iron via the chlorination of a mixture containing iron(III) oxide by ammonium chloride with the subsequent decomposition of the intermediate product to iron(III) chloride, its sublimation, and further reduction in a hydrogen flow was proposed. The reaction between ammonium chloride and iron(III) oxide and the thermal decomposition of the ammonium chloride complex were thermogravimetrically studied. The conditions and energetic characteristics of these reactions were determined. The method was tested on iron-containing raw materials. The impurities in the end product of reduction by hydrogen were 0.25%.
Similar content being viewed by others
References
A. Willmes, Taschenbuch chemische Substanzen (Harri Deutsch, Frankfurt (M.), 2007).
O. V. Grineva, A. N. Dyachenko, and R. I. Kraydenko, Russ. J. Appl. Chem. 86, 339 (2013).
T. K. Mukerjee and C. K. Gupta, Int. J. 1, 111 (1983).
Chul Tae Lee, Young Hong Ryoo, Woong Ki Kang, and Young-Hyun Paik, J. Kor. Inst. Chem. Eng. 25, 1 (1985).
R. S. Zhu, J. H. Wang, and M. C. Lin, J. Phys. Chem. 111, 13831 (2007).
C. S. M. Partiti and F. O. Jorge, Hyperfine Interact. 110, 121 (1997).
Y. Calage, J. L. Dormann, M. C. Moron, and F. Palacio, Hyperfine Interact. 54, 483 (1990).
S. R. Brown, M. Attenborough, I. Hall, and O. Nikolov, Solid State Commun. 95, 787 (1995).
N. W. Gregory, Inorg. Chem. 20, 3661 (1981).
H. Lepp, Am. Mineral. 425, 679 (1957).
Z. Liu and Y. Zheng, Powder Technol. 209, 119 (2011).
S. Blairs, J. Chem. Thermodyn. 38, 1484 (2006).
Hwa Young Lee and Sung Gyu Kim, Powder Technol. 152, 16 (2005).
Rigg Tyson, Can. J. Chem. Eng. 42, 247 (1964.
Masahito Uchikoshi, Junichi Imaizumi, Hideka Shibuya, et al., Thin Solid Films 461, 94 (2004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. D’yachenko, R.I. Kraidenko, A.A. Smorokov, 2018, published in Zhurnal Neorganicheskoi Khimii, 2018, Vol. 63, No. 6, pp. 700–705.
Rights and permissions
About this article
Cite this article
D’yachenko, A.N., Kraidenko, R.I. & Smorokov, A.A. Preparation of High-Purity Iron by Means of Ammonium Chloride Complexes. Russ. J. Inorg. Chem. 63, 736–741 (2018). https://doi.org/10.1134/S0036023618060074
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023618060074