Abstract
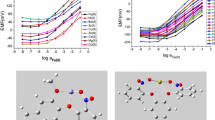
Ion-selective properties of polymeric plasticized membranes of ion-selective electrodes based on 1,2-bis[2-(2-diphenylphosphorylmethylphenoxy)ethoxy]benzene (L1) and 1,2-bis[2-(2-diphenylphosphorylphenoxy)ethoxy]benzene (L2) toward certain bivalent metal cations has been studied. It has been shown that podand L2 can be used as a sensor component of polymeric plasticized membrane of ion-selective electrode for quantitative determination of Pb2+ ions in aqueous solutions. The structure of complexes [CdI2(μ-L1)]2, [Cd(μ-L1)Br2]2 · C2H5OH, and [Cd(L2)2(H2O)2][Cd2I6] has been studied by X-ray diffraction and IR spectroscopy.
Similar content being viewed by others
References
O. M. Petrukhin, E. V. Shipulo, S. A. Krylova, et al., Zh. Anal. Khim. 49, 1299 (1994).
M. Yu. Nemilova, N. V. Shvedene, V. L. Filimonova, et al., Zh. Anal. Khim. 49, 418 (1994).
N. V. Shvedene, T. V. Shishkanova, V. E. Baulin, et al., Vestn. Mosk. Univ., Ser. 2: Khim. 37, 273 (1996).
O. M. Petrukhin, S. N. Kurachenkova, E. A. Sonina, et al., J. Anal. Chem. 57, 313 (2002).
E. S. Krivorot’ko, I. N. Polyakova, I. S. Ivanova, et al., Russ. J. Inorg. Chem. 61, 1241 (2016). doi 10.1134/S0036023616100132
N. I. Movchan, N. N. Umarova, R. A. Yusupov, et al., RF Patent No. 2152609 (1999).
E. N. Pyatova, A. N. Kopytin, E. G. Il’in, et al., RF Patent No. 2054666 (1996).
D. O. Kirsanov, A. V. Legin, V. A. Babain, et al., RF Patent No. 2315988 (2006).
E. N. Pyatova, I. N. Polyakova, I. S. Ivanova, et al., Russ. J. Inorg. Chem. 62, 431 (2017).
L. Kh. Minacheva, I. S. Ivanova, I. K. Kireeva, et al., Zh. Neorg. Khim. 39, 1143 (1994).
L. Kh. Minacheva, I. S. Ivanova, I. K. Kireeva, et al., Crystallogr. Rep. 45, 52 (2000).
I. S. Ivanova, I. N. Polyakova, V. E. Baulin, et al., Russ. J. Gen. Chem 86, 865 (2016). doi 10.1134/S1070363216040186
V. E. Baulin, I. S. Ivanova, I. N. Polyakova, et al., Russ. J. Gen. Chem. 85, 899 (2015). doi 10.1134/S1070363215040234
APEX2, SAINT, SADABS (Bruker AXS Inc., Madison, Wisconsin, 2008–2009).
G. M. Sheldrick, Acta Crystallogr. 64, 112 (2008).
L. Palatinus and G. Chapius, J. Appl. Crystallogr. 40, 786 (2007).
L. Palatinus, S. J. Prathapa, and S. van Smaalen, J. Appl. Crystallogr. 45, 575 (2012).
IUPAC. Recommendation for Nomenclature of Ion Selective Electrodes, Pure Appl. Chem. 48, 127 (1976).
E. Bakker, E. Pretsch, and P. Buhlmann, Anal. Chem. 72, 1127 (2000).
R. C. Hayward, C. H. Overton, and G. H. Whitham, J. Chem. Soc., Perkin Trans. 1, 2413 (1976).
X.-J. Yang, X. Liu, Y. Liu, et al., Polyhedron 29, 934 (2010).
K. Sreevani, K. Thangaraj, K. Ramamurthi, et al., J. Cryst. Growth 322, 78 (2011).
I. A. Zamilatskov, E. V. Savinkina, and D. V. Al’bov, Russ. J. Coord. Chem. 33, 396 (2007).
E. V. Savinkina, I. A. Zamilatskov, E. A. Buravlev, and D. V. Albov, Mendeleev Commun. 18, 131 (2008).
C. C. Hines, W. M. Reichert, S. T. Griffin, et al., J. Mol. Struct. 796, 76 (2006).
G.-Ch. Xu, Y.-J. Ding, T. Okuamura, et al., Cryst. Eng. Comm. 10, 1052 (2008).
R. L. Oriolli and M. Clampolini, Chem. Commun. 1280 (1972).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.S. Ivanova, I.N. Polyakova, E.S. Krivorot’ko, E.N. Pyatova, V.E. Baulin, A.Yu. Tsivadze, 2017, published in Zhurnal Neorganicheskoi Khimii, 2017, Vol. 62, No. 10, pp. 1308–1316.
Rights and permissions
About this article
Cite this article
Ivanova, I., Polyakova, I., Krivorot’ko, E. et al. Ion-selective properties of 1,2-bis[2-(2-diphenylphosphorylmethylphenoxy)ethoxy]benzene (L1) and 1,2-bis[2-(2-diphenylphosphorylphenoxy)ethoxy]benzene (L2). Crystal structure of complexes [CdI2(μ-L1)]2, [CdBr2 (μ-L1)]2 · C2H5OH, and [Cd(L2)2(H2O)2][Cd2I6]. Russ. J. Inorg. Chem. 62, 1299–1307 (2017). https://doi.org/10.1134/S0036023617100102
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023617100102