Abstract—
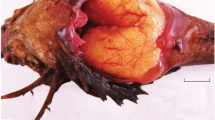
Oocyte morphology and spermatozoa ultrastructure are studied in the representatives of the genera Parascorpaena and Scorpaenopsis. Oocytes of P. aurita and S. cirrosa are attached to peduncles originated from ovigerous stroma located in the central part of the ovary. Oocyte diameter at the final phases of vitellogenesis is 450 and 500 µm, respectively. The oogenesis is asynchronous and continuous. Spermatozoa of four species (P. aurita, P. mossambica, S. cirrosa, and S. diabolus) possess similar morphology: spermatozoon head width is slightly larger than its length. The genera Parascorpaena (three species) and Scorpaenopsis (four species) differ in three from four indices designating spermatozoon head and midpiece shapes, size of nuclear fossa in the spermatozoon nucleus base (20–25 vs. 0–12% of head length), and relative location of proximal and distal centrioles (coaxial and orthogonal, respectively). The irregular shape of the nuclear fossa in the spermatozoon nucleus base of P. aurita can be interpreted as apomorphy.




Similar content being viewed by others
Notes
Ten individuals of P. mossambica 11.2–14.0 cm TL have been recently described from the coastal zone (Visakhapatnam) of India in the stony area covered with water vegetation at a depth of 12 m (Naranji et al., 2017). A presence of reddish-yellow ocular tentacles on the head of the fish is mentioned, but these structures usual for the species are not described as diagnostic characters, and they are not shown in the figure. Therefore, correct identification of the fishes seems problematic.
REFERENCES
Allen, G.R. and Erdmann, M.V., Reef Fishes of the East Indies, Vols. 1–3: Tropical Reef Research, Perth: Univ. of Hawaii Press, 2012.
Baccetti, B. and Afzelius, B.A., The Biology of the Sperm Cell, Monogr. Dev. Biol. vol. 10, College Station, TX, 1976.
Emel’yanova, N.G. and Makeeva, A.P., Morphophysiological features of spermatozoa of some cyprinids and catfishes, in Problemy reproduktivnoi biologii v trudakh professora S.I. Kulaeva i ego posledovatelei (The Works on Reproductive Biology of Professor S.I. Kulaev and His Followers), Moscow: Mosk. Gos. Univ., 1998, pp. 260−269.
Emel’yanova, N.G. and Pavlov, D.A., Some data on reproductive biology of spotted ghoul Inimicus sinensis (Synanceiidae), J. Ichthyol., 2020, vol. 60, no. 3, pp. 453–461. https://doi.org/10.1134/S0032945220030078
Emel’yanova, N.G. and Pavlov, D.A., Features of reproductive biology of Papuan scorpionfish Scorpaenopsis papuensis (Scorpaenidae), J. Ichthyol., 2021, vol. 61, no. 1, pp. 143–151. https://doi.org/10.1134/S0032945221010069
Eschmeyer’s Catalog of Fishes: Genera, Species, References, Version 01/2020, Fricke R., Eschmeyer W.N., and van der Laan R., Eds., 2020. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
FAO Species Identification Guide for Fishery Purposes. The Living Marine Resources of the Western Central Pacific, Vol. 4: Bony Fishes, Part 2: Mugilidae to Carangidae, Carpenter, K.E. and Niem, V.H., Eds., Rome: UN Food Agric. Org., 1999, pp. 2069–2790.
França, G.F., Oliveira, C., and Quagio-Grassiotto, I., Spermatic cell characteristics in Gymnotiformes (Teleostei: Ostariophysi) and their phylogenetic meaning, J. Fish Biol., 2009, vol. 75, pp. 2226–2243. https://doi.org/10.1111/j.1095-8649.2009.02439.x
FishBase, Version 12/2019, Froese, R. and Pauly, D., Eds., 2019. http://www.fishbase.org.
García-López, A., Martínez-Rodríguez, G., and Sarasquete, C., Male reproductive system in Senegalese sole Solea senegalensis (Kaup), Histol. Histopathol., 2005, vol. 20, no. 4, pp. 1179–1189. https://doi.org/10.14670/HH-20.1179
Giora, J. and Burns, J.R., Sperm ultrastructure in three different families of weakly electric fishes (Teleostei: Gymnotiformes), Neotrop. Ichthyol., 2011, vol. 9, no. 4, pp. 881–888.
Götting, K.J., Beitrage zur Kenntnis der Grundlagen. der Fortpflanzung und zur. Fruchtbarkeitbestimmung bei marinen Teleosteern, Helgol. Wiss. Meeresunters., 1961, vol. 8, no. 1, pp. 1–41.
Imamura, H., Phylogenetic relationships and new classification of the superfamily Scorpaenoidea (Actinopterygii: Perciformes), Species Diversity, 2004, vol. 9, pp. 1–36.
Ishida M., Phylogeny of the suborder Scorpaenoidei (Pisces: Scorpaeniformes), Bull. Nansei Nat. Fish. Res. Inst., 1994, vol. 27, pp. 1–112.
Jamieson, B.G.M., Fish Evolution and Systematics: Evidence from Spermatozoa: With a Survey of Lophophorate, Echinoderm and Protochordate Sperm and an Account of Gamete Cryopreservation, Cambridge: Cambridge Univ. Press, 1991.
Koya, Y. and Muñoz, M., Comparative study on ovarian structures in scorpaenids: possible evolutional process of reproductive mode, Ichthyol. Res., 2007, vol. 54, pp. 221–230. https://doi.org/10.1007/s10228-006-0394-7
Kuiter, R.H. and Tonozuka, T., Pictorial Guide to Indonesian Reef Fishes, Part 1: Eels–Snappers, Muraenidae–Lutjanidae, Seaford, VC: Zoonetics, 2001.
Kwik, J.T.B., The biology and ecology of small tropical scorpaenoids inhabiting shallow coastal habitats in Singapore, PhD Thesis, Singapore: Nat. Univ. of Singapore, 2011. http://scholarbank.nus.sg/handle/10635/31654.
Lahnsteiner, F. and Patzner, R.A., Spermiogenesis and structure of mature spermatozoa in bleniid fishes (Pisces, Bleniidae), J. Submicrosc. Cytol. Pathol., 1990, vol. 22, pp. 565–576.
Lieske, E. and Myers, R., Collins Pocket Guide. Coral Reef Fishes. Indo-Pacific and Caribbean Including the Red Sea, London: Harper Collins, 1994.
Magalhaes, A.L.B., Andrade, R.F., Gomes, B.V.C., et al., Ultrastructure of the semicystic spermatogenesis in the South American freshwater characid Hemigrammus marginatus (Teleostei, Characiformes), J. Appl. Ichthyol., 2011, vol. 27, no. 4, pp. 1041–1046. https://doi.org/10.1111/j.1439-0426.2011.01747.x
Masuda, H., Amaoka, K., Araga, C., et al., The Fishes of the Japanese Archipelago, Tokyo: Tokai Univ. Press, 1984, vol. 1.
Mattei, X., Spermatozoon ultrastructure and its systematic implication in fishes, Can. J. Zool., 1991, vol. 69, pp. 3038–3055. https://doi.org/10.1139/z91-428
Mattei, X., Peculiarities in the organization of testis of Ophidion sp. (Pisces: Teleostei). Evidence for two types of spermatogenesis in teleost fish, J. Fish Biol., 1993, vol. 43, no. 6, pp. 931–937. https://doi.org/10.1006/jfbi.1993.1196
Moser, H.G., Reproduction and development of Sebastodes paucispinis and comparison with other rock fishes of Southern California, Copeia, 1967, no. 4, pp. 773–797.
Muñoz, M., Casadevall, M., and Bonet, S., Testicular structure and semicystic spermatogenesis in a specialized ovuliparous species: Scorpaena notata (Pisces, Scorpaenidae), Acta Zool. (Stockholm), 2002a, vol. 83, no. 3, pp. 213–219. https://doi.org/10.1046/j.1463-6395.2002.00114.x
Muñoz, M., Casadevall, M., and Bonet, S., The ovarian morphology of Scorpaena notata shows a specialized mode of oviparity, J. Fish. Biol., 2002b, vol. 61, no. 4, pp. 877–887. https://doi.org/10.1111/j.1095-8649.2002.tb01849.x
Naranji, M.K., Velamala, G.R., and Sujatha, K., New record of Mozambique scorpionfish, Parascorpaena mossambica (Peters, 1855), (Actinopterygii: order, Scorpaeniformes; family, Scorpaenidae) from Indian waters, Indones. J. Mar. Sci., 2017, vol. 22, no. 3, pp. 105–110. https://doi.org/10.14710/ik.ijms.22.3.105-110
Oven, L.S., Spetsifika razvitiya polovykh kletok morskikh ryb v period razmnozheniya kak pokazatel’ tipa neresta i reaktsii na usloviya sredy obitaniya (Specific Development of Oocytes of Marine Fishes during Reproduction as an Indicator of Spawning Type and Reaction on Environmental Conditions), Moscow: VNIRO, 2004.
Pavlov, D.A., Otolith morphology and relationships of several fish species of the suborder Scorpaenoidei, J. Ichthyol., 2021, vol. 61, no. 1, pp. 33–47. https://doi.org/10.1134/S0032945221010100
Pavlov, D.A. and Emel’yanova, N.G., Reproductive biology of species from the family Scorpaenidae and transition from oviparity to viviparity in the southern and northern Percomorpha, in Viviparous Fishes II, Uribe, M.C. and Grier, H.J., Eds., Homestead: New Life, 2010, pp. 89–105.
Pavlov, D.A. and Emel’yanova, N.G., Comparative analysis of spermatozoa morphology in three fish species from the suborder Scorpaenoidei, J. Ichthyol., 2018, vol. 58, no. 2, pp. 226–238. https://doi.org/10.1134/S003294521802011X
Pavlov, D.A. and Emel’yanova, N.G., Biological characteristics of Dendrochirus zebra (Cuvier, 1829) (Scorpaeniformes: Scorpaenidae) from Nha Trang Bay, South China Sea, Russ. J. Mar. Biol., 2019, vol. 45, no. 2, pp. 75–85. https://doi.org/10.1134/S106307401902010X
Pecio, A., Spermiogenesis and fine structure of the spermatozoon in a headstander, Chilodus punctatus (Teleostei, Characiformes, Anostomidae), Folia Biol., 2003, vol. 51, pp. 55–62.
Randall, J.E. and Eschmeyer, W.N., Revision of the Indo-Pacific scorpionfish genus Scopaenopsis, with descriptions of eight new species, Indo-Pac. Fish., 2001, no. 34.
Roskin, G.I. and Levinson, L.B., Mikroskopicheskaya tekhnika (Methods of Microscopy), Moscow: Sovetskaya Nauka, 1957.
Sàbat, M., Lo Nostro, F., Casadevall, M., and Muñoz, M., A light and electron microscopic study on the organization of the testis and the semicystic spermatogenesis of the genus Scorpaena (Teleostei, Scorpaenidae), J. Morphol., 2009, vol. 270, no. 6, pp. 662–672. https://doi.org/10.1002/jmor.10707
Santhanam, R., Biology and Ecology of Venomous Marine Scorpionfishes, London: Academic, 2019.
Shahin, A.A.B., Semicystic spermatogenesis and biflagellate spermatozoon ultrastructure in the Nile electric catfish Malapterurus electricus (Teleostei: Siluriformes: Malapteruridae), Acta Zool., 2006, vol. 87, no. 3, pp. 215–227. https://doi.org/10.1111/j.1463-6395.2006.00235.x
Shaw, F.R., Morado, J.F., Lowe, V.C., and McDermott, S.F., An Atlas of Reproductive Development in Rockfishes, Genus Sebastes, NOAA Prof. Pap. NMFS no. 14, Silver Spring: Natl. Ocean. Atmos. Adm., 2012.
Smith, W.L. and Wheeler, W.C., Polyphyly of the mail-cheeked fishes (Teleostei: Scorpaeniformes): evidence from mitochondrial and nuclear sequence data, Mol. Phylogenet. Evol., 2004, vol. 32, no. 2, pp. 627–646. https://doi.org/10.1016/j.ympev.2004.02.006
Smith, W.L. and Wheeler, W.C., Venom evolution widespread in fishes: a phylogenetic road map for the bioprospecting of piscine venoms, J. Hered., 2006, vol. 97, no. 3, pp. 206–217. https://doi.org/10.1093/jhered/esj034
Smith, W.L., Everman, E., and Richardson, C., Phylogeny and taxonomy of flatheads, scorpionfishes, sea robins, and stonefishes (Percomorpha: Scorpaeniformes) and the evolution of the lachrymal saber, Copeia, 2018, vol. 106, no. 1, pp. 94–119. https://doi.org/10.1643/CG-17-669
Spadella, M.A., Oliveira, C., and Quagio-Grassiotto, I., Comparative analysis of spermiogenesis and sperm ultrastructure in Callichthyidae (Teleostei: Ostariophysi: Siluriformes), Neotrop. Ichthyol., 2007, vol. 5, no. 3, pp. 337–350. https://doi.org/10.1590/S1679-62252007000300014
Takahashi, H., Takano, K., and Takemura, A., Reproductive cycles of Sebastes taczanowskii, compared with those of other rockfishes of the genus Sebastes, Environ. Biol. Fish., 1991, vol. 30, pp. 23–30. https://doi.org/10.1007/BF02296873
Vila, S., Muñoz, M., Sabat, M., and Casadevall, M., Annual cycle of stored spermatozoa within the ovaries of Helicolenus dactylopterus dactylopterus (Teleostei, Scorpaenidae), J. Fish Biol., 2007, vol. 71, pp. 596–609. https://doi.org/10.1111/j.1095-8649.2007.01525.x
Vila, S., Sàbat, M., Muñoz M., and Casadevall, M., Spermiogenesis particularities of a sperm storage species: Helicolenus dactylopterus (Teleostei: Scorpaenidae), Sci. Mar., 2010, vol. 74, no. 4, pp. 687–704. https://doi.org/10.3989/scimar.2010.74n4697
Weakley, B.S., Beginner’s Handbook in Biological Electron Microscopy, Edinburgh: Churchill Livingstone, 1972.
Wourms, J.P., Reproduction and development of Sebastes in the context of the evolution of piscine viviparity, Environ. Biol. Fish., 1991, vol. 30, pp. 111–126. https://doi.org/10.1007/978-94-011-3792-8_12
Wourms, J.P., Grove, B.D., and Lombardi, J., The maternal embryonic relationships in viviparous fishes, in Fish Physiology, Hoar, W.S. and Randall, D.J., Eds., San Diego: Academic, 1988, vol. 11B, pp. 1–134.
ACKNOWLEDGMENTS
We thank Vo Thi Ha and Dinh Thị Hai Yen (Coastal Department of the Russian–Vietnamese Tropical Research and Technological Center) for help in the collection of the material. We thank A.M. Prokofiev (Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences) for help in fish identification.
Funding
The study was carried out with financial support from the Russian–Vietnamese Tropical Research and Technological Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflict of interest. Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by D. Pavlov
Rights and permissions
About this article
Cite this article
Emel’yanova, N.G., Pavlov, D.A. Features of Oogenesis and Spermatozoa Ultrastructure in the Fishes of the Genera Parascorpaena and Scorpaenopsis (Scorpaenidae). J. Ichthyol. 61, 912–922 (2021). https://doi.org/10.1134/S0032945221050040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945221050040