Abstract
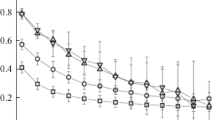
In order to determine the most efficient modes of copper-nanoparticle synthesis, a number of experiments on evaporation with the subsequent condensation of the initial material in the argon atmosphere have been carried out. In the course of the experiments, it has been discovered that intensified evaporation significantly increases the average size of the synthesized particles. However, the investigation of the change in the dimensional characteristics of the produced clusters depending on the intensity of the buffer-gas flow faced serious difficulties. The obtained results differ significantly from the earlier experiments on the synthesis of the transition-metal oxides. In order to solve this contradiction, the computer simulation of the condensation of copper atoms from the gas phase with three different cooling rates and two final temperatures T = 373 K and Т = 77 K has been performed. It has been discovered that the cooling rate of the gas mixture and the final temperature directly influence the quantity and size of the produced particles. Thus, at a tenfold lower cooling rate, the average number of particles increases 2.7 times at a final temperature of 77 K and by 3.1 times at T = 373 K.
Similar content being viewed by others
References
L. V. Zolotukhina, B. R. Gel’chinskii, N. V. Kishkoparov, S. A. Oglezneva, and D. V. Ershov, “Nanodispersed copper powders: Production, properties and applications,” Nanotekhnika, No. 8, 22–26 (2006).
K. K. R. Datta, C. Kulkarni, and M. Eswaramoorthy, “Aminoclay: A permselective matrix to stabilize copper nanoparticles,” Chem. Commun. 46, 616–618 (2010).
A. G. Nasibulin, L. I. Shurygina, and E. I. Kauppinen, “Synthesis of nanoparticles using vapor-phase decomposition of copper (II) acetylacetonate,” Colloid J. 67, 1–20 (2005).
R. Strobel and S. E. Pratsinis, “Flame aerosol synthesis of smart nanostructured materials,” J. Mater. Chem. 17, 4743–4756 (2007).
N. Osterwalder, C. Capello, K. Hungerbuhler, and W. J. Stark, “Energy consumption during nanoparticle production: How economic is dry synthesis?” J. Nanopart. Res. 8, 1–9 (2006).
A. P. Zav’yalov, K. V. Zobov, I. K. Chakin, V. V. Syzrantsev, and S. P. Bardakhanov, “Synthesis of copper nanopowders using electron-beam evaporation at atmospheric pressure of inert gas,” Nanotechnology in Russia 9, 660–666 (2014).
P. A. Lin, A. Kumar, and R. M. Sankaran, “New insights into plasma-assisted dissociation of organometallic vapors for gas-phase synthesis of metal nanoparticles,” Plasma Proces. Polym. 9, 1184–1193 (2012).
A. P. Weber, P. Davoodi, M. Seipenbusch, and G. Kasper, “Size effects in the catalytic activity of unsupported metallic nanoparticles,” J. Nanopart. Res. 5, 293–298 (2003).
A. P. Weber, M. Seipenbusch, C. Thanner, and G. Kasper, “Aerosol catalysis on nickel,” J. Nanopart. Res. 1, 253–265 (1999).
H. Fissan, M. K. Kennedy, T. J. Krinke, and F. E. Kruis, “Nanoparticles from the gas phase as building blocks for electrical devices,” J. Nanopart. Res. 5, 299–310 (2003).
S. V. Zhidovinova, L. V. Zolotukhina, and B. R. Gel’chinskii, “Oxidation of copper ultrafine powders and nanopowders produced by vapor phase condensation,” Russ. Metall. (Metally) 2010, 774–778 (2010).
A. A. Rempel’, “Nanotechnologies. Properties and applications of nanostructured materials,” Russ. Chem. Rev. 76, 435–461 (2007).
Heerman, D.V., Computer Simulations Methods in Theoretical Physics (Springer-Verlag, Berlin, 1986).
B. J. Alder and T. E. Wainwright, “Molecular dynamics computations for the hard sphere system,” Nuovo Cim. 9, 116–132 (1958).
A. N. Lagar’kov and V. M. Sergeev, “Molecular dynamics method in statistical physics,” Phys.-Usp. 21, 566–588 (1978).
V. A. Polukhin, V. F. Ukhov, and M. M. Dzugutov, Computer Simulation of Dynamics and Structures of Liquid Crystals (Nauka, Moscow, 1981) [in Russian].
K. Binder and D. W. Heerman, Monte-Carlo Simulations in Statistical Physics (Springer-Verlag, Berlin, 1992; Nauka, Moscow, 1995).
K. Ohno, K. Esfarjani, and Y. Kawazoe, Computational materials science. From ab-initio to Monte Carlo methods (Springer-Verlag, Berlin, 1999).
V. A. Polukhin and E. D. Kurbanova, “Dependence of the thermal stability of the interface states of d metals (Cu, Pd, Ti, Ni) and Al with graphene on the character of sorption and diffusion mobility in a contact zone,” Russ. J. Phys. Chem. A 89, 531–546 (2015).
A. E. Galashev and V. A. Polukhin, “Computer analysis of the stability of copper films on graphene,” Russ. J. Phys. Chem. A 88, 995–999 (2014).
V. S. Myasnichenko and M. D. Starostenkov, “Formation of fivefold axes in the FCC-metal nanoclusters,” Appl. Surf. Sci. 260, 51–53 (2012).
V. A. Polukhin and N. A. Vatolin, “Stability and technical evolution of transition-metal and silicon clusters,” Russ. Chem. Rev. 84, 498–539 (2015).
A. G. Vorontsov, B. R. Gel’chinskii, and A. E. Korenchenko, “Kinetics and energy states of nanoclusters in the initial stage of homogeneous condensation at high supersaturation degrees,” J. Exp. Theor. Phys. 115, 789–797 (2012).
M. S. Daw and M. I. Baskes, “Embedded-atom method: Derivation and application to impurities, surfaces and other defects in metals,” Phys. Rev. B: Condens. Matter 29, 6443–6453 (1984).
E. Kesälä, A. Kuronen, and K. Nordlund, “Molecular dynamics simulation of pressure dependence of cluster growth in inert gas condensation,” Phys. Rev. B: Condens. Matter Mate. Phys. 75, 174121 (2007).
V. Rosato, M. Guillope, and B. Legrand, “Thermodynamical and structural properties of FCC transition metals using a simple tight-binding model,” Philos. Mag. A 59, 321–336 (1989).
F. H. Stillinger and T. A. Weber, “Computer simulation of local order in condensed phases of silicon,” Phys. Rev. B: Condens. Matter 31, 5262–5271 (1985).
F. Cleri and V. Rosato, “Tight-binding potentials for transition metals and alloys,” Phys. Rev. B: Condens. Matter 48, 22–33 (1993).
S. L. Gafner and Yu. Ya. Gafner, “Analysis of gasphase condensation of nickel nanoparticles,” J. Exp. Theor. Phys. 107, 712–722 (2008).
J. F. Ziegler, J. P. Biersack, and U. Littmark, The Stopping and Range of Ions in Solids (Pergamon, New York, 1985).
K. Yasuoka and X. C. Zeng, “Molecular dynamics of homogeneous nucleation in the vapor phase of Lennard-Jones. III. Effect of carrier gas pressure,” J. Chem. Phys. 126, 124320 (2007).
K. Yasuoka and M. J. Matsumoto, “Molecular dynamics of homogeneous nucleation in the vapor phase. I. Lennard-Jones fluid,” J. Chem. Phys. 109, 8451–8463 (1998).
J. D. Honeycutt and H. C. Anderson, “Molecular dynamics study of melting and freezing of small Lennard-Jones clusters,” J. Chem. Phys. 91, 4950–4963 (1987).
S. Tsyganov, J. Kastner, B. Rellinghaus, T. Kauffeldt, F. Westerhoff, and D. Wolf, “Analysis of Ni nanoparticle gas phase sintering,” Phys. Rev. B: Condens. Matter Mater. Phys. 75, 045421 (2007).
P. Krasnechtchekov, K. Albe, and R. S. Averback, “Simulations of the inert gas condensation process,” Z. Metallkd. 94, 1098–1105 (2003).
I. V. Chepkasov, Yu. Ya. Gafner, and S. L. Gafner, “Effect of cooling rate and final temperature on the structure and form of copper nanoclusters synthesized from gas phase,” Pis’ma o Mater. 1, 107–109 (2011).
N. Luemmen and T. Kraska, “Investigation of the formation of iron nanoparticles from the gas phase by molecular dynamics simulations,” Nanotecnology 15, 525–533 (2004).
N. B. Volkov, E. L. Fen’ko, and A. P. Yalovets, “Simulation of generation of ultradispersed particles upon irradiation of metals by a high-power electron beam,” Tech. Phys. 55, 1389–1399 (2010).
V. M. Ievlev and E. V. Shvedov, “Kinetics of formation of discrete nanostructures during vacuum condensation from a single-component vapor,” Phys. Solid State 48, 144–149 (2006).
G. Rollmann, R. Meyer, and P. Entel, “Nucleation and growth of Ni clusters in an Ar atmosphere,” Phase Trans. 78, 733–740 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.V. Chepkasov, Yu.Ya. Gafner, S.L. Gafner, S.P. Bardahanov, 2016, published in Fizika Metallov i Metallovedenie, 2016, Vol. 117, No. 10, pp. 1037–1047.
Rights and permissions
About this article
Cite this article
Chepkasov, I.V., Gafner, Y.Y., Gafner, S.L. et al. Condensation of Cu nanoparticles from the gas phase. Phys. Metals Metallogr. 117, 1003–1012 (2016). https://doi.org/10.1134/S0031918X16080020
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X16080020