Abstract
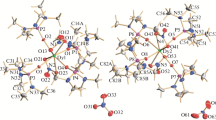
The crystal structure of the [DyCl2(H2O)6]Cl complex is studied by X-ray diffraction analysis. The crystals are constructed of complex cations [DyCl2(H2O)6]+ and outer-sphere Cl−1 ions. The structure is represented by separate [DyCl2(H2O)6] complexes bound by a dense network of \({\text{O}{-}\text{H}}\cdots{\text{Cl}}\) hydrogen bonds. The coordination polyhedron of Dy(III) with coordination number 8 is represented by a weakly distorted square antiprism. The results of investigation of the luminescent properties of the complex are presented.





Similar content being viewed by others
REFERENCES
Triboluminescence. Theory, Synthesis, and Application, Ed. by D. O. Olawale, O. O. I. Okoli, R. S. Fontenot, and W. A. Hollerman (Springer, Switzerland, 2016), p. 39. www.springer.com/la/book/9783319388410.
Z. M. Hao, G. C. Yang, X. Z. Song, M. Zhu, X. Meng, S. N. Zhao, S. Y. Song, and H. J. Zhang, J. Mater. Chem. A 2, 237 (2014). https://doi.org/10.1039/C3TA13179C
A. G. Mirochnik, N. V. Petrochenkova, A. S. Shishov, B. V. Bukvetskii, T. B. Emelina, A. A. Sergeev, and S. S. Voznesenskii, Spectrochim. Acta A 155, 111 (2016). https://doi.org/10.1016/j.saa.2015.11.004
J-C. G. Bunzli, Chem. Rev. 110, 2729 (2010). https://doi.org/10.1021/cr900362e
J-C. G. Bunzli and S. V. Eliseeva, Chem. Sci. 4, 1939 (2013). https://doi.org/10.1039/C3SC22126A
U. Hommerich, E. Nyein, J. A. Freeman, P. Amedzake, S. B. Trivedi, and J. M. J. Zavada, J. Cryst. Growth 287, 230 (2006). https://doi.org/10.1016/j.jcrysgro.2005.11.019
J. Feng, L. Zhou, S. Y. Song, Z. F. Li, W. Q. Fan, L. N. Sun, Y. N. Yu, and H. J. Zhang, Dalton Trans., 6593 (2009). https://doi.org/10.1039/b906419b
K. Wie, D. P. Machewirth, J. Wenzal, E. Snitzer, and G. H. Sigel, Opt. Lett. 19, 904 (1994). https://doi.org/10.1364/OL.19.000904
X. D. Huang, Y. Xu, K. Fan, S. S. Bao, M. Kurmoo, and L. M. Zheng, Angew. Chem. 57, 8577 (2018). https://doi.org/10.1002/anie.201804102
M. Venkataravanappa, R. B. Basavaraj, G. P. Darshan, B. D. E. Prasad, S. C. Sharma, P. H. Prabha, and H. Nagabhushana, J. Rare Earths 36, 690 (2018). https://doi.org/10.1016/j.jre.2017.11.013
W. A. Dar, Z. Ahmed, and K. Iftikhar, J. Photochem. Photobiol., A 356, 502 (2018). https://doi.org/10.1016/j.jphotochem.2017.12.017
J. H. Wang, S. Chorazy, K. Nakabayashi, B. Sieklucka, and S. Ohkoshi, J. Mater. Chem. C 6, 473 (2018). https://doi.org/10.1039/C7TC03963H
Q. Su, Z. Pei, L. Chi, H. Zhang, Z. Zhang, and F. J. Zou, J. Alloys Compd. 192, 25 (1993). https://doi.org/10.1016/0925-8388(93)90174-L
X. Liu, Y. Liu, D. Yan, H. Zhu, C. Liu, C. Xu, Y. Liu, and X. Wang, J. Mater. Chem. 22, 16839 (2012). https://doi.org/10.1039/C2JM32741D
B. V. Bukvetskii, A. G. Mirochnik, and P. A. Zhikhareva, Opt. Spectrosc. 126, 195 (2019). https://doi.org/10.1134/S0030400X19030044
Bruker, SMART and SAINT-Plus. Versions 5.0, Data Collection and Processing Software for the SMART System (Bruker AXS Inc., Madison, WI, USA, 1998).
G. M. Sheldrick, SHELXTL/PC. Versions 5. 10. An Integrated System for Solving, Refining and Displaying Crystal Structures From Diffraction Data (Bruker AXS Inc., Madison, WI, USA, 1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Basieva
Rights and permissions
About this article
Cite this article
Bukvetskii, B.V., Mirochnik, A.G. & Zhikhareva, P.A. Crystal Structure and Luminescence of the [DyCl2(H2O)6]Cl Complex. Opt. Spectrosc. 128, 323–326 (2020). https://doi.org/10.1134/S0030400X20030054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X20030054