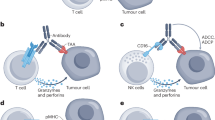
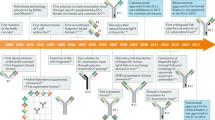
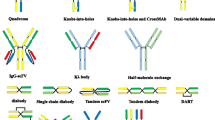
Abstract
Bispecific antibodies capable of simultaneously binding two targets have been studied for many years with a view to their implementation in clinical practice. Unique biological and pharmacological properties, as well as the diversity of their formats, make it possible to consider bispecific antibodies as promising agents for use in various procedures: from visualization of intracellular processes to targeted anticancer therapy. Bispecific antibodies help to determine more precisely the therapeutic target, thereby increasing the efficiency of therapy and reducing the probability of side effects. The present review describes the main formats of bispecific antibodies, methods for their generation, and possibilities for practical application.
Similar content being viewed by others
Abbreviations
- BsAb:
-
bispecific antibodies
- scFv:
-
single chain antibody variable fragment
- MICA:
-
major histocompatibility complex class I chain-related protein A
- ММР:
-
matrix metalloproteinases
- TNF:
-
tumor necrosis factor
- HER2 and HER3:
-
human epidermal growth factor receptors
- RA:
-
rheumatoid arthritis
- HCC:
-
hepatocellular carcinoma
References
Nisonoff A., Rivers M.M. 1961. Recombination of a mixture of univalent antibody fragments of different specificity. Arch. Biochem. Biophys. 93, 460–467.
Kontermann R.E. 2005. Recombinant bispecific antibodies for cancer therapy. Acta Pharmacol. Sin. 26 (1), 1–9.
Milstein C., Cuello A.C. 1983. Hybrid hybridomas and their use in immunohistochemistry. Nature. 305 (5934), 537–540.
Suresh M.R., Cuello A.C., Milstein C. 1986. Advantages of bispecific hybridomas in one-step immunocytochemistry and immunoassays. Proc. Natl. Acad. Sci. U. S. A. 83 (20), 7989–7993.
Reinagel M.L., Taylor R.P. 2000. Transfer of immune complexes from erythrocyte CR1 to mouse macrophages. J. Immunol. 164 (4), 1977–1985.
Helfrich W., Bremer E. 2014. Bifunctional antibody fragment-based fusion proteins for the targeted elimination of pathogenic T-cell subsets. In: Systemic Lupus Erythematosus: Methods and Protocols. Methods in Molecular Biology, vol. 1134. Eds. Eggleton P., Ward F.J. New York: Humana Press, pp. 79–93.
Bremer E., Abdulahad W.H., de Bruyn M., et al. 2011. Selective elimination of pathogenic synovial fluid T-cells from rheumatoid arthritis and juvenile idiopathic arthritis by targeted activation of Fas-apoptotic signaling. Immunol. Lett. 138, 161–168.
Farrington G.K., Caram-Salas N., Haqqani A.S., et al. 2014. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J. 28, 4764–4778.
Stanimirovic D., Kemmerich K., Haqqani A.S., Farrington G.K. 2014. Engineering and pharmacology of blood–brain barrier-permeable bispecific antibodies. Adv. Pharmacol. 71, 301–335.
Nettelbeck D.M., Rivera A.A., Kupsch J., et al. 2004. Retargeting of adenoviral infection to melanoma: Combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int. J. Cancer. 108, 136–145.
Bachanova V., Frankel A.E., Cao Q., et al. 2015. Phase 1 study of a bispecific ligand-directed toxin targeting CD22 and CD19 (DT2219) for refractory B-cell malignancies. Clin. Cancer Res. 21 (6), 1267–1272.
Cheal S.M., Yoo B., Boughdad S., et al. 2014. Evaluation of glycodendron and synthetically modified dextran clearing agents for multistep targeting of radioisotopes for molecular imaging and radioimmunotherapy. Mol. Pharmaceutics. 11 (2), 400–416.
Stamova S., Feldmann A., Cartellieri M., et al. 2012. Generation of single-chain bispecific green fluorescent protein fusion antibodies for imaging of antibodyinduced T cell synapses. Anal. Biochem. 423, 261–268.
Chames P., Baty D. 2009. Bispecific antibodies for cancer therapy. mAbs. 1 (6), 539–547. doi 10.4161/mabs.1.6.10015
Lee R.J., Fang Q., Davol P.A., et al. 2007. Antibody targeting of stem cells to infarcted myocardium. Stem Cells. 25, 712–717.
Spiess C., Zhai Q., Carter P.J. 2015. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol. Immunol. 67 (2), 95–106.
Yang F., Wen W., Qin W. 2016. Bispecific antibodies as a development platform for new concepts and treatment strategies. Int. J. Mol. Sci. 18 (1), 48.
Schaefer W., Völger H.R., Lorenz S., et al. 2016. Heavy and light chain pairing of bivalent quadroma and knobs-into-holes antibodies analyzed by UHR-ESIQTOF mass spectrometry. mAbs. 8 (1), 49–55. doi 10.1080/19420862.2015.1111498
Ridgway J.B., Presta L.G., Carter P. 1996. “Knobsinto-holes” engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 9 (7), 617–621.
Von Kreutenstein T.S., Escobar-Carbrera E., Lario P.I., et al. 2013. Improving biophysical properties of a bispecific antibody scaffold to aid developability: Quality by molecular design. mAbs. 5, 646–654.
Davis J.H., Aperlo C., Li Y., Kurosawa E., et al. 2010. SEEDbodies: Fusion proteins based on strandexchange engineered domain (SEED) CH3 heterodimers in an Fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng., Des. Sel. 23, 195–202.
Kontermann R.E. 2012. Dual targeting strategies with bispecific antibodies. mAbs. 4, 182–197.
Spasevska I., Duong M.N., Klein C., Dumontet C. 2015. Advances in bispecific antibodies engineering: Novel concepts for immunotherapies. J. Blood Disord. Transfus. 6, 243.
Schaefer W., Regula J.T., Bähner M., et al. 2011. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc. Natl. Acad. Sci. U. S. A. 108, 11187–11192.
Lewis S.M., Wu X., Pustilnik A., et al. 2014. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat. Biotechnol. 32, 191–198.
Castoldi R., Jucknischke U., Pradel L.P., et al. 2012. Molecular characterization of novel trispecific ErbBcMet-IGF1R antibodies and their antigen-binding properties. Protein Eng., Des. Sel. 25, 551–560.
Schanzer J., Jekle A., Nezu J., et al. 2011. Development of tetravalent, bispecific CCR5 antibodies with antiviral activity against CCR5 monoclonal antibody-resistant HIV-1 strains. Antimicrob. Agents Chemother. 55 (5), 2369–2378.
Brockmann E.C., Cooper M., Stromsten N., et al. 2005. Selecting for antibody scFv fragments with improved stability using phage display with denaturation under reducing conditions. J. Immunol. Methods, 296, 159–170.
Miller B.R., Demarest S.J., Lugovskoy A., et al. 2010. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng., Des. Sel. 23 (7), 549–557.
Manzke O., Tesch H., Diehl V., Bohlen H. 1997. Single-step purification of bispecific monoclonal antibodies for immunotherapeutic use by hydrophobic interaction chromatography. J. Immunol. Methods. 208 (1), 65–73.
Müller-Späth Th., Ulmer N., Aumann L., et al. 2013. Purifying common light-chain bispecific antibodies: A twin-column, countercurrent chromatography platform process. BioProcess Int. 11 (5), 36–45.
Kontermann R.E., Brinkmann U. 2015. Bispecific antibodies. Drug Discovery Today. 20 (7), 838–847.
Eigenbrot C., Fuh G. 2013. Two-in-one antibodies with dual action Fabs. Curr. Opin. Chem. Biol. 17 (3), 400–405.
Wu C., Ying H., Bose S., et al. 2009. Molecular construction and optimization of anti-human IL-1α/β dual variable domain immunoglobulin (DVD-IgTM) molecules. mAbs. 1 (4), 339–347.
Labrijn A.F., Meesters J.I., Priem P., et al. 2014. Controlled Fab-arm exchange for the generation of stable bispecific IgG1. Nat. Protoc. 9 (10), 2450–2463.
Fischer N., Elson G., Magistrelli G., et al. 2015. Exploiting light chains for the scalable generation and platform purification of native human bispecific IgG. Nat. Commun. 6, 6113.
Le Gall F., Kipriyanov S.M., Moldenhauer G., Little M. 1999. Di-, tri-and tetrameric single chain Fv antibody fragments against human CD19: Effect of valency on cell binding. FEBS Lett. 453 (1–2), 164–168.
Wu M.-R., Zhang T., Gacerez A.T., et al. 2015. B7H6-specific bispecific T cell engagers (BiTEs) lead to tumor elimination and host anti-tumor immunity. J. Immunol. 194 (11), 5305–5311.
Bargou R., Leo E., Zugmaier G., et al. 2008. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 321, 974–977.
Moore P.A., Zhang W., Rainey G.J., et al. 2011. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 117 (17), 4542–4551.
Sharkey R.M., Rossi E.A., McBride W.J., et al. 2010. Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin. Nucl. Med. 40 (3), 190–203. doi 10.1053/j.semnuclmed.2009.12.002
Rossi E.A., Rossi D.L., Cardillo T.M., et al. 2011. Preclinical studies on targeted delivery of multiple IFN-α2b to HLA-DR in diverse hematologic cancers. Blood. 118, 1877–1884.
Bodet-Milin C., Ferrer L., Rauscher A., et al. 2015. Pharmacokinetics and dosimetry studies for optimization of pretargeted radioimmunotherapy in CEAexpressing advanced lung cancer patients. Front. Med. (Lausanne). 2, 84.
Deev S.M., Lebedenko E.N. 2009. Modern technologies for creating synthetic antibodies for clinical application. Acta Naturae. 1, 32–50.
Revets H., Baetselier P.D., Muyldermans S. 2005. Nanobodies as novel agents for cancer therapy. Expert Opin. Biol. Ther. 5 (1), 111–124.
Conrath K.E., Lauwereys M., Wyns L., Muyldermans S. 2001. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 276 (10), 7346–7350.
Marschall A.L.J., Dübel S., Böldicke T. 2015. Specific in vivo knockdown of protein function by intrabodies. mAbs. 7 (6), 1010–1035.
Jendreyko N., Popkov M., Rader C., Barbas C.F. 3rd. 2005. Phenotypic knockout of VEGF-R2 and Tie-2 with an intradiabody reduces tumor growth and angiogenesis in vivo. Proc. Natl. Acad. Sci. U. S. A. 102, 8293–8298.
Huston J.S., Levinson D., Mudgett-Hunter M., et al. 1988. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin singlechain Fv analogue produced in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 85, 5879–5883.
Hust M., Jostock T., Menzel C., et al. 2007. Single chain Fab (scFab) fragment. BMC Biotechnol. 7, 14.
Colby D.W., Garg P., Holden T., et al. 2004. Development of a human light chain variable domain (V(L)) intracellular antibody specific for the amino terminus of huntingtin via yeast surface display. J. Mol. Biol. 342, 901–912.
Kim D.S., Song H.N., Nam H.J., et al. 2014. Directed evolution of human heavy chain variable domain (VH) using in vivo protein fitness filter. PLoS One. 9, e98178.
Fishburn C.S. 2008. The pharmacology of PEGylation: Balancing PD with PK to generate novel therapeutics. J. Pharm. Sci. 97 (10), 4167–4183.
Merlot A.M., Kalinowski D.S., Kovacevic Z., et al. 2015. Making a case for albumin—a highly promising drug-delivery system. Future Med. Chem. 7 (5), 553–556.
Fan G., Wang Z., Hao M., Li J. 2015. Bispecific antibodies and their applications. J. Hematol. Oncol. 8, 130.
Taki S., Kamada H., Inoue M., et al. 2015. A novel bispecific antibody against human CD3 and ephrin receptor A10 for breast cancer therapy. PLoS One. 10 (12), e0144712.
Gschwind A., Fischer O.M., Ullrich A. 2004. The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer. 4 (5), 361–370.
Adams G.P., Weiner L.M. 2005. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 23, 1147–1157.
Sharkey R.M., Goldenberg D.M. 2006. Targeted therapy of cancer: New prospects for antibodies and immunoconjugates. CA Cancer J. Clin. 56, 226–243.
Asano R., Shimomura I., Konno S., et al. 2014. Rearranging the domain order of a diabodybased IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics. mAbs. 6 (5), 1243–1254.
Shen Y., Zeng L., Novosyadlyy R., et al. 2015. A bifunctional antibody-receptor domain fusion protein simultaneously targeting IGF-IR and VEGF for degradation. mAbs. 7 (5), 931–945.
Lu D., Zhang H., Ludwig D., et al. 2004. Simultaneous blockade of both the epidermal growth factor receptor and the insulin-like growth factor receptor signaling pathways in cancer cells with a fully human recombinant bispecific antibody. J. Biol. Chem. 279, 2856–2865.
Rubinfeld B., Upadhyay A., Clark S.L., et al. 2006. Identification and immunotherapeutic targeting of antigens induced by chemotherapy. Nat. Biotechnol. 24, 205–209.
Lugovskoy A.A. 2017. Engineering antibodies as drugs: Principles and practice. Mol. Biol. (Moscow). 51 (6), 772–781.
Breton C.S., Nahimana A., Aubry D., et al. 2014. A novel anti-CD19 monoclonal antibody (GBR 401) with high killing activity against B cell malignancies. J. Hematol. Oncol. 7 (1), 33.
Mullard A. 2014. FDA approves first bispecific. Nat. Rev. Drug Discov. 14 (1), 7.
Queudeville M., Handgretinger R., Ebinger M. 2017. Immunotargeting relapsed or refractory precursor Bcell acute lymphoblastic leukemia: Role of Blinatumomab. OncoTargets Ther. 10, 3567–3578.
Topp M.S., Gokbuget N., Stein A.S. 2015. Safety and activity of Blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 16, 57–66.
Kantarjian H., Stein A., Gokbuget N. 2017. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 376, 836–847.
Bellone S., Black J., English D.P., et al. 2016. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE®), is highly active against primary uterine serous papillary carcinoma cell lines in vitro. Am. J. Obstet. Gynecol. 214 (1), 99.e1–99.e8.
Leong S.R., Sukumaran S., Hristopoulos M., et al. 2017. An anti-CD3/anti-CLL-1 bispecific antibody for the treatment of acute myeloid leukemia. Blood. 129 (5), 609–618.
Wu J., Fu J., Zhang M., Liu D. 2015. AFM13: A firstin-class tetravalent bispecific anti-CD30/CD16A antibody for NK cell-mediated immunotherapy. J. Hematol. Oncol. 8 (1), 96.
Wang T., Sun F., Xie W., et al. 2016. A bispecific protein rG7S-MICA recruits natural killer cells and enhances NKG2D-mediated immunosurveillance against hepatocellular carcinoma. Cancer Lett. 372, 166–178.
Hirschhaeuser F., Leidig T., Rodday B., et al. 2009. Test system for trifunctional antibodies in 3D MCTS culture. J. Biomol. Screening. 14 (8), 980–990.
Riechelmann H., Wiesneth M., Schauwecker P., et al. 2007. Adoptive therapy of head and neck squamous cell carcinoma with antibody coated immune cells: A pilot clinical trial. Cancer Immunol. Immunother. 56, 1397–1406.
Stanglmaier M., Faltin M., Ruf P., et al. 2008. Bi20 (FBTA05), a novel trifunctional bispecific antibody (anti-CD20 × anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. Int. J. Cancer. 123, 1181–1189
Lindhofer H., Hess J., Ruf P. 2011. Trifunctional Triomab ® antibodies for cancer therapy. In: Bispecific Antibodies. Ed. Kontermann R.E. Berlin: Springer-Verlag, 289–312.
Seimetz D., Lindhofer H., Bokemeyer C. 2010. Development and approval of the trifunctional antibody Catumaxomab (anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer Treat. Rev. 36 (6), 458–467.
Ruf P., Jager M., Ellwart J., et al. 2004. Two new trifunctional antibodies for the therapy of human malignant melanoma. Int. J. Cancer. 108, 725–732.
Jäger M., Schoberth A., Ruf P., et al. 2009. The trifunctional antibody Ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor 2. Cancer Res. 69, 4270–4276.
Heiss M.M., Murawa P., Koralewski P., et al. 2010. The trifunctional antibody Catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int. J. Cancer. 27 (9), 2209–2221.
Kiewe P., Hasmüller S., Kahlert S., et al. 2006. Phase I trial of the trifunctional anti-HER2 × anti-CD3 antibody Ertumaxomab in metastatic breast cancer. Clin. Cancer Res. 12 (10), 3085–3091.
Stanglmaier M., Faltin M., Ruf P., et al. 2008. Bi20 (fBTA05), a novel trifunctional bispecific antibody (anti-CD20 × anti-CD3), mediates efficient killing of B-cell lymphoma cells even with very low CD20 expression levels. Int. J. Cancer. 123 (5), 1181–1189.
Koristka S., Cartellieri M., Theil A., et al. 2012. Retargeting of human regulatory T cells by single-chain bispecific antibodies. J. Immunol. 188, 1551–1558.
Lahdenranta J., Paragas V., Kudla A.J., et al. 2013. Preclinical activity of MM-111, a bispecific ErbB2/ErbB3 antibody in previously treated ErbB2-positive gastric and gastroesophageal junction cancer. J. Clin. Oncol. 31 (Suppl. 4), 48. doi 10.1200/jco.2013.31.4_suppl.48
Fitzgerald J.B., Johnson B.W., Baum J., et al. 2014. MM-141, an IGF-IR-and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol. Cancer Ther. 13 (2), 410–425.
Reid A., Vidal L., Shaw H., de Bono J. 2007. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur. J. Cancer. 43, 481–489.
Guo X.-F., Zhu X.-F., Yang W.-C., et al. 2014. An EGFR/HER2-bispecific and enediyne-energized fusion protein shows high efficacy against esophageal cancer. PLoS One. 9 (3), e92986.
Geyer C.E., Forster J., Lindquist D., et al. 2006. Lapatinib plus Capecitabine for HER2-positive advanced breast cancer. N. Eng. J. Med. 355, 2733–2743.
Ding L., Tian C., Feng S., et al. 2015. Small sized EGFR1 and HER2 specific bifunctional antibody for targeted cancer therapy. Theranostics. 5 (4), 378–398.
Biel N.M., Siemann D.W. 2016. Targeting the angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Lett. 380 (2), 525–533.
Baker L.C., Boult J.K., Thomas M., et al. 2016. Acute tumour response to a bispecific Ang-2-VEGF-A antibody: Insights from multiparametric MRI and gene expression profiling. Br. J. Cancer. 115 (6), 691–702.
DiGiandomenico A., Keller A.E., Gao C., et al. 2014. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 6 (262), 262ra155.
Rossotti M.A., González-Techera A., Guarnaschelli J., et al. 2015. Increasing the potency of neutralizing single-domain antibodies by functionalization with a CD11b/CD18 binding domain. mAbs. 7 (5), 820–828.
Sun M., Pace C.S, Yao X., et al. 2014. Rational design and characterization of the novel, broad and potent bispecific HIV-1 neutralizing antibody iMabm36. J. Acquired Immune Defic. Syndr. 66 (5), 473–483.
Jiang X., Jia Q., Lu L., et al. 2016. A novel bispecific peptide HIV-1 fusion inhibitor targeting the N-terminal heptad repeat and fusion peptide domains in gp41. Amino Acids. 48, 2867–2873.
Chen W., Feng Y., Prabakaran P., et al. 2014. Exceptionally potent and broadly cross-reactive, bispecific multivalent HIV-1 inhibitors based on single human CD4 and antibody domains. J. Virol. 88 (2), 1125–1139.
Yu X., Duval M., Gawron M., et al. 2016. Overcoming the constraints of anti-HIV/CD89 bispecific antibodies that limit viral inhibition. J. Immunol. Res. 2016, 9425172.
Shi X., Deng Y., Wang H., et al. 2016. A bispecific antibody effectively neutralizes all four serotypes of dengue virus by simultaneous blocking virus attachment and fusion. mAbs. 8 (3), 574–584.
Geoghegan E.M., Zhang H., Desai P.J., et al. 2015. Antiviral activity of a single-domain antibody immunotoxin binding to glycoprotein D of herpes simplex virus 2. Antimicrob. Agents Chemother. 59 (1), 527–535.
Ibanez L.I., de Filette M., Hultberg A., et al. 2011. Nanobodies with in vitro neutralizing activity protect mice against H5N1 influenza virus infection. J. Infect. Dis. 203 (8), 1063–1072.
Manicourt D.H., Fujimoto N., Obata K., Thonar E.J. 1995. Levels of circulating collagenase, stromelysin-1, and tissue inhibitor of matrix metalloproteinases 1 in patients with rheumatoid arthritis: Relationship to serum levels of antigenic keratan sulfate and systemic parameters of inflammation. Arthritis Rheumatol. 38, 1031–1039.
Silva L.C., Ortigosa L.C., Benard G. 2010. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: Mechanisms of action and pitfalls. Immunotherapy. 2, 817–833.
Rubbert-Roth A. 2012. Assessing the safety of biologic agents in patients with rheumatoid arthritis. Rheumatology. 51, 38–47.
Alzabin S., Abraham S.M., Taher T.E., et al. 2012. Incomplete response of inflammatory arthritis to TNFα blockade is associated with the Th17 pathway. Ann. Rheum. Dis. 71, 1741–1748.
Fischer J.A., Hueber A.J., Wilson S., et al. 2015. Combined inhibition of tumor necrosis factor α and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis development and characterization of a novel bispecific antibody. Arthritis Rheumatol. 67 (1), 51–62.
Genovese M.C., Weinblatt M., Aelion J.A., et al. 2016. ABT-122, a Tnf–and IL-17–targeted dual variable domain (DVD)–Ig™ in rheumatoid arthritis patients with inadequate response to methotrexate: Results from a phase 2 trial. ACR/ARHP Annual Meeting, Washington, DC. Arthritis Rheumatol. 68 (Suppl. 10).
Qi J., Kan F., Ye X., et al. 2012. A bispecific antibody against IL-1β and IL-17A is beneficial for experimental rheumatoid arthritis. Int. Immunopharmacol. 14, 770–778.
Bootz F., Neri D. 2016. Immunocytokines: A novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discovery Today. 21 (1), 180–189.
Hughes C., Sette A., Seed M., et al. 2014. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res. Ther. 16 (4), R151.
Efimov G.A., Kruglov A.A., Khlopchatnikova Z.V., et al. 2016. Cell-type-restricted anti-cytokine therapy: TNF inhibition from one pathogenic source. Proc. Natl. Acad. Sci. U. S. A. 113 (11), 3006–3011.
Wu C., Ying H., Grinnell C., et al. 2007. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat. Biotechnol. 25, 1290–1297.
DiGiammarino E.L., Harlan J.E., Walter K.A., et al. 2011. Ligand association rates to the inner-variabledomain of a dual-variable-domain immunoglobulin are significantly impacted by linker design. mAbs. 3, 487–494.
Onuoha S.C., Ferrari M., Sblattero D., Pitzalis C. 2015. Rational design of antirheumatic prodrugs specific for sites of inflammation. Arthritis Rheumatol. 67 (10), 2661–2672.
Liu M., Xie M., Jiang S., et al. 2014. A novel bispecific antibody targeting tumor necrosis factor α and ED-B fibronectin effectively inhibits the progression of established collagen-induce arthritis. J. Biotechnol. 186, 1–12.
Kruglov A.A., Lampropoulou V., Fillatreau S., Nedospasov S.A. 2011. Pathogenic and protective functions of TNF in neuroinflammation are defined by its expression in T lymphocytes and myeloid cells. J. Immunol. 187, 5660–5670.
Winsauer C., Kruglov A.A., Chashchina A.A., et al. 2014. Cellular sources of pathogenic and protective TNF and experimental strategies based on utilization of TNF humanized mice. Cytokine Growth Factor Rev. 25 (2), 115–123.
Mokhonov V.V., Shilov E.S., Korneev K.V., et al. 2016. Novel bispecific proteins binding cytokines and myeloid cell surface markers. Ross. Immunol. Zh. 10 (19), 378–385.
Bremer E., ten Cate B., Samplonius D.F., et al. 2006. CD7-restricted activation of Fas-mediated apoptosis: A novel therapeutic approach for acute T-cell leukemia. Blood. 107, 2863–2870.
Wilk E., Witte T., Marquardt N., et al. 2009. Depletion of functionally active CD20+ T cells by Rituximab treatment. Arthritis Rheumatol. 60, 3563–3571.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.A. Vasilenko, V.V. Mokhonov, E.N. Gorshkova, I.V. Astrakhantseva, 2018, published in Molekulyarnaya Biologiya, 2018, Vol. 52, No. 3, pp. 380–393.
Rights and permissions
About this article
Cite this article
Vasilenko, E.A., Mokhonov, V.V., Gorshkova, E.N. et al. Bispecific Antibodies: Formats and Areas of Application. Mol Biol 52, 323–334 (2018). https://doi.org/10.1134/S0026893318020176
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893318020176