Abstract
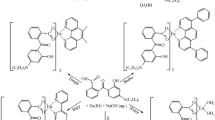
By the interaction of CuI with bis(2-pyridyl)phosphine oxides (2-Py)2RP(O) containing E-1-phenyl-2-aroylethenyl–C(Ph)=CHC(O)R′ (R′ = Ph, 2-thienyl) substituents, [Cu2I2L2] complexes are synthesized. Their structures are based on the rhomboidal Cu2I2 moiety whose copper atoms are N,N′-chelated by the indicated ligands. At room temperature, the crystalline samples of these complexes exhibit dark red photoluminescence (λmax = 680-685 nm) with a charge transfer from the Cu2I2 moiety to the ligand.






Similar content being viewed by others
REFERENCES
R. Czerwieniec, M. J. Leitl, H. H. H. Homeier, and H. Yersin. Coord. Chem. Rev., 2016, 325, 2-28. https://doi.org/10.1016/j.ccr.2016.06.016
J. Troyano, F. Zamora, and S. Delgado. Chem. Soc. Rev., 2021, 50, 4606-4628. https://doi.org/10.1039/D0CS01470B
C. E. Housecroft and E. C. Constable. J. Mater. Chem. C, 2022, 10, 4456-4482. https://doi.org/10.1039/D1TC04028F
W. Liu, Y. Fang, and J. Li. Adv. Funct. Mater., 2018, 28, 1705593. https://doi.org/10.1002/adfm.201705593
M. F. Galimova, E. M. Zueva, A. B. Dobrynin, I. E. Kolesnikov, R. R. Musin, E. I. Musina, and A. A. Karasik. Dalton Trans., 2021, 50, 13421-13429. https://doi.org/10.1039/D1DT02344F
T. S. Sukhikh, R. M. Khisamov, and S. N. Konchenko. Molecules, 2021, 26, 2030. https://doi.org/10.3390/molecules26072030
C. Kirst, J. Tietze, P. Mayer, H.-C. Böttcher, and K. Karaghiosoff. ChemistryOpen, 2022, 11, e202100224. https://doi.org/10.1002/open.202100224
A. M. Khalil, C. Xu, V. Delmas, G. Calvez, K. Costuas, M. Haouas, and C. Lescop. Inorg. Chem. Front., 2021, 8, 4887-4895.
F. Moutier, J. Schiller, G. Calvez, and C. Lescop. Org. Chem. Front., 2021, 8, 2893-2902. https://doi.org/10.1039/D1QO00538C
C. Lescop. Chem. Rec., 2020, 21, 544-557.
S. Evariste, A. M. Khalil, S. Kerneis, C. Xu, G. Calvez, K. Costuas, and C. Lescop. Inorg. Chem. Front., 2020, 7, 3402-3411. https://doi.org/10.1039/D0QI00691B
N. A. Shekhovtsov, T. E. Kokina, K. A. Vinogradova, A. Y. Panarin, M. I. Rakhmanova, D. Y. Naumov, N. V. Pervukhina, E. B. Nikolaenkova, V. P. Krivopalov, R. Czerwieniec, and M. B. Bushuev. Dalton Trans., 2022, 51, 2898-2911. https://doi.org/10.1039/D1DT04325K
K. A. Vinogradova, V. P. Krivopalov, E. B. Nikolaenkova, N. V. Pervukhina, D. Y. Naumov, E. G. Boguslavsky, and M. B. Bushuev. Dalton Trans., 2016, 45, 515-524. https://doi.org/10.1039/C5DT04005A
K. A. Vinogradova, V. F. Plyusnin, A. S. Kupryakov, M. I. Rakhmanova, N. V. Pervukhina, D. Yu. Naumov, L. A. Sheludyakova, E. B. Nikolaenkova, V. P. Krivopalov, and M. B. Bushuev. Dalton Trans., 2014, 43, 2953-2960. https://doi.org/10.1039/C3DT53040J
Y.-Q. Chen, G.-R. Li, Z. Chang, Y.-K. Qu, Y.-H. Zhang, and X.-H. Bu. Chem. Sci., 2013, 4, 3678-3682. https://doi.org/10.1039/C3SC00057E
X. Wang, X. Tian, Q. Zhang, P. Sun, J. Wu, H. Zhou, B. Jin, J. Yang, S. Zhang, C. Wang, X. Tao, M. Jiang, and Y. Tian. Chem. Mater., 2012, 24, 954-961. https://doi.org/10.1021/cm2029855
K. Tsugea, Y. Chishina, H. Hashiguchi, Y. Sasaki, M. Kato, S. Ishizaka, and N. Kitamura. Coord. Chem. Rev., 2016, 306, 636-651. https://doi.org/10.1016/j.ccr.2015.03.022
S. N. Arbuzova, N. K. Gusarova, T. E. Glotova, I. A. Ushakov, S. I. Verkhoturova, A. O. Korocheva, and B. A. Trofimov. Eur. J. Org. Chem., 2014, 639-643. https://doi.org/10.1002/ejoc.201301453
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox. Gaussian09, Revision C.01. Wallingford, CT: Gaussian, 2010.
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch. J. Chem. Phys., 1994, 98, 11623-11627. https://doi.org/10.1021/j100096a001
P. J. Hay and W. R. Wadt. J. Chem. Phys., 1985, 82, 299-310. https://doi.org/10.1063/1.448975
G. M. Sheldrick. SADABS: Program for Empirical Absorption Correction of Area Detector Data. Göttingen, Germany: University of Göttingen, 2002.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
A. Bondi. Phys. Chem., 1964, 68, 441-451. https://doi.org/10.1021/j100785a001
A. V. Artemev, M. R. Ryzhikov, I. V. Taidakov, M. I. Rakhmanova, E. A. Varaksina, I. Yu. Bagryanskaya, S. F. Malysheva, and N. A. Belogorlova. Dalton Trans., 2018, 47, 2701-2710. https://doi.org/10.1039/C7DT04758D
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation (projects Nos. 121031700321-3, 121021000199-6) using the material and facilities of the Baikal Analytical Multi-Access Center, Siberian Branch, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 9, 99099.https://doi.org/10.26902/JSC_id99099
Supplementary material
Rights and permissions
About this article
Cite this article
Arbuzova, S.N., Verkhoturova, S.I., Borodina, T.N. et al. CuI COMPLEXES BASED ON DI(2-PYRIDYL) (2-AROYLETHENYL)PHOSPHINE OXIDES: SYNTHESIS, STRUCTURE, AND DARK RED PHOTOLUMINESCENCE. J Struct Chem 63, 1383–1389 (2022). https://doi.org/10.1134/S0022476622090013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622090013