Abstract
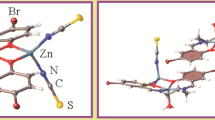
A new zinc(II) complex of 2-(((2-((2-hydroxyethyl)amino)ethyl)imino)methyl)phenol (L), [Zn(Lz)Br2] (1), is prepared and identified by elemental analysis, FTIR and 1H NMR spectroscopy, and single crystal X-ray diffraction. The X-ray structure analysis of 1 reveals a tetrahedrally coordinated zinc(II) complex containing the NO-donor amino alcoholic Schiff base ligand and two bromo ligands. After complexation, the ligand (L) converts to its zwitterionc form (Lz) of phenol → phenolate; amine → ammonium. In this structure, hydrogen bonds between amine and alcohol units form different types of hydrogen bond motifs, including \(\text{R}_{2}^{1}(7)\), \(\text{R}_{2}^{2}(7),\ \text{R}_{2}^{2}(10),\ \text{R}_{4}^{4}(24),\ \text{R}_{4}^{4}(30),\ \text{R}_{6}^{6}(38)\), and \(\text{R}_{6}^{6}(44)\). In addition to the hydrogen bonds in this crystal network, there are π–π stacking interactions between the phenyl ring and the imine group. The ability of the ligand and its isostructural complexes [Zn(Lz)Br2] (1), [Zn(Lz)Cl2] (2), and [Zn(Lz)I2] (3) to interact with ten selected biomacromolecules (BRAF kinase, CatB, DNA gyrase, HDAC7, rHA, RNR, TrxR, TS, Top II, and B-DNA) is investigated by docking studies. The results show that in some cases, the studied compound can interact with proteins and DNA better than doxorubicin. The charge distribution pattern of the ligand and complex 1 is studied by the NBO analysis.







Similar content being viewed by others
REFERENCES
E. Fullam, A. Abuhammad, D. L. Wilson, M. C. Anderton, S. G. Davies, A. J. Russell, and E. Sim. Bioorg. Med. Chem. Lett., 2011, 21, 1185. https://doi.org/10.1016/j.bmcl.2010.12.099
R. Yendapally and R. E. Lee. Bioorg. Med. Chem. Lett., 2008, 18, 1607. https://doi.org/10.1016/j.bmcl.2008.01.065
H. X. Wei, D. Lu, V. Sun, J. Zhang, Y. Gu, P. Osenkowski, W. Ye, D. J. Selkoe, M. S. Wolfe, and C. E. Augelli-Szafran. Bioorg. Med. Chem. Lett., 2016, 26, 2133. https://doi.org/10.1016/j.bmcl.2016.03.042
I. Declerck, B. Himpens, G. Droogmans, and R. Casteels. Pflügers Arch., 1990, 417, 117. https://doi.org/10.1007/BF00370780
M. M. Weinberger. Pediatr. Clin. North Am., 1975, 22, 121. https://doi.org/10.1016/S0031-3955(16)33107-8
S. J. Kwon and S. Y. Ko. Tetrahedron Lett., 2002, 43, 639. https://doi.org/10.1016/S0040-4039(01)02206-7
W. H. Frishman. Circulation, 2003, 107, e117. https://doi.org/10.1161/01.CIR.0000070983.15903.A2
D. T. Nash. Clin. Cardiol., 1990, 13, 764. https://doi.org/10.1002/clc.4960131104
Z. Mardani, R. Kazemshoar-Duzduzani, K. Moeini, A. Hajabbas-Farshchi, C. Carpenter-Warren, A. M. Z. Slawin, and J. D. Woollins. RSC Adv., 2018, 8, 28810. https://doi.org/10.1039/C8RA04578J
Z. Mardani, M. Hakimi, K. Moeini, and F. Mohr. Acta Crystallogr., Sect. C, 2019, 75, 951. https://doi.org/10.1107/S2053229619008258
K. Chennakesava Rao, Y. Arun, K. Easwaramoorthi, C. Balachandran, T. Prakasam, T. Eswara Yuvaraj, and P. T. Perumal. Bioorg. Med. Chem. Lett., 2014, 24, 3057. https://doi.org/10.1016/j.bmcl.2014.05.027
S. Vanguru, L. Jilla, Y. Sajja, R. Bantu, L. Nagarapu, J. B. Nanubolu, B. Bhaskar, N. Jain, S. Sivan, and V. Manga. Bioorg. Med. Chem. Lett., 2017, 27, 792. https://doi.org/10.1016/j.bmcl.2017.01.031
M. Zhang, D.-M. Xian, H.-H. Li, J.-C. Zhang, and Z.-L. You. Aust. J. Chem., 2012, 65, 343. https://doi.org/10.1071/CH11424
M. D. Altintop, A. Özdemir, G. Turan-Zitouni, S. Ilgin, Ö. Atli, G. İşcan, and Z. A. Kaplancikli. Eur. J. Med. Chem., 2012, 58, 299. https://doi.org/10.1016/j.ejmech.2012.10.011
V. C. Da Silveira, J. S. Luz, C. C. Oliveira, I. Graziani, M. R. Ciriolo, and A. M. D. C. Ferreira. J. Inorg. Biochem. 2008, 102, 1090. https://doi.org/10.1016/j.jinorgbio.2007.12.033
K. Shanker, R. Rohini, V. Ravinder, P. M. Reddy, and Y.-P. Ho. Spectrochim. Acta, Part A, 2009, 73, 205. https://doi.org/10.1016/j.saa.2009.01.021
H.-W. Lin. Synth. React. Inorg. Nano-Met. Chem., 2009, 39, 73. https://doi.org/10.1080/15533170902762595
M. Taha, N. H. Ismail, M. S. Baharudin, S. Lalani, S. Mehboob, K. M. Khan, S. Yousuf, S. Siddiqui, F. Rahim, and M. I. Choudhary. Med. Chem. Res., 2015, 24, 1310. https://doi.org/10.1007/s00044-014-1213-8
C. Jing, C. Wang, K. Yan, K. Zhao, G. Sheng, D. Qu, F. Niu, H. Zhu, and Z. You. Bioorg. Med. Chem., 2016, 24, 270. https://doi.org/10.1016/j.bmc.2015.12.013
J. R. Morrow and K. A. Kolasa. Inorg. Chim. Acta, 1992, 195, 245. https://doi.org/10.1016/S0020-1693(00)85319-0
A. D. Tiwari, A. K. Mishra, S. B. Mishra, B. B. Mamba, B. Maji, and S. Bhattacharya. Spectrochim. Acta, Part A, 2011, 79, 1050. https://doi.org/10.1016/j.saa.2011.04.018
E. M. Hodnett and W. J. Dunn. J. Med. Chem., 1970, 13, 768. https://doi.org/10.1021/jm00298a054
E. M. Hodnett and W. J. Dunn. J. Med. Chem., 1972, 15, 339. https://doi.org/10.1021/jm00273a037
V. R. Martínez, M. V. Aguirre, J. S. Todaro, O. E. Piro, G. A. Echeverría, E. G. Ferrer, and P.a.M. Williams. Toxicol. In Vitro, 2018, 48, 205. https://doi.org/10.1016/j.tiv.2018.01.009
A. Adhikari, N. Kumari, M. Adhikari, N. Kumar, A. K. Tiwari, A. Shukla, A. K. Mishra, and A. Datta. Bioorg. Med. Chem., 2017, 25, 3483. https://doi.org/10.1016/j.bmc.2017.04.035
J. Dam, Z. Ismail, T. Kurebwa, N. Gangat, L. Harmse, H. M. Marques, A. Lemmerer, M. L. Bode, and C. B. De Koning. Eur. J. Med. Chem., 2017, 126, 353. https://doi.org/10.1016/j.ejmech.2016.10.041
L. Saghatforoush, K. Moeini, S. A. Hosseini-Yazdi, Z. Mardani, A. Hajabbas-Farshchi, H. T. Jameson, S. G. Telfer, and J. D. Woollins. RSC Adv., 2018, 8, 35625. https://doi.org/10.1039/C8RA07463A
F. Marandi, K. Moeini, F. Alizadeh, Z. Mardani, C. K. Quah, W.-S. Loh, and J. D. Woollins. Inorg. Chim. Acta, 2018, 482, 717-725. https://doi.org/10.1016/j.ica.2018.07.014
F. Marandi, K. Moeini, F. Alizadeh, Z. Mardani, C. K. Quah, and W.-S. Loh. Z. Naturforsch. B, 2018, 73, 369-375. https://doi.org/10.1515/znb-2018-0043
F. Marandi, K. Moeini, A. Arkak, Z. Mardani, and H. Krautscheid. J. Coord. Chem., 2018, 71, 3893-3911. https://doi.org/10.1080/00958972.2018.1543871
Z. Mardani, V. Golsanamlou, Z. Jabbarzadeh, K. Moeini, S. Khodavandegar, C. Carpenter-Warren, A. M. Z. Slawin, and J. D. Woollins. J. Coord. Chem., 2018, 71, 4109-4131. https://doi.org/10.1080/00958972.2018.1536268
L. Saghatforonsh, M. Hakimi, A. Gholipour, A. Bakhtiary, K. Moeini, V. Eigner, and M. Duek. Polyhedron, 2021, 208, 115440. https://doi.org/10.16/i.poly.2021.115440
M. Hakimi, Z. Mardani, and K. Moeini. J. Chem. Res., 2013, 37, 140. https://doi.org/10.3184/174751913X13575773628099
M. Hakimi, Z. Mardani, K. Moeini, E. Schuh, and F. Mohr. Z. Naturforsch. B, 2013, 68, 272. https://doi.org/10.5560/znb.2013-2295
M. Hakimi, Z. Mardani, K. Moeini, and F. Mohr. Polyhedron, 2015, 102, 569. https://doi.org/10.1016/j.poly.2015.10.038
F. H. Allen. Acta Crystallogr., Sect. B, 2002, 58, 380-388. https://doi.org/10.1107/S0108768102003890
M. Hakimi, Z. Mardani, K. Moeini, F. Mohr, M. A. Fernandes. Polyhedron, 2014, 67, 27-35. https://doi.org/10.1016/j.poly.2013.08.065
M. Hakimi, K. Moeini, Z. Mardani, and F. Mohr. Polyhedron, 2014, 70, 92. https://doi.org/10.1016/j.poly.2013.12.033
F. Marandi, K. Moeini, B. Mostafazadeh, and H. Krautscheid. Polyhedron, 2017, 133, 146-154. https://doi.org/10.1016/j.poly.2017.05.029
M. Hakimi, H. Rezaei, K. Moeini, Z. Mardani, V. Eigner, and M. Dušek. J. Mol. Struct., 2020, 1207, 127804. https://doi.org/10.1016/j.molstruc.2020.127804
F. Marandi, K. Moeini, Z. Mardani, and H. Krautscheid. Acta Crystallogr., Sect. C, 2019, 75, 1023. https://doi.org/10.1107/S2053229619008301
Z. Mardani, V. Golsanamlou, S. Khodavandegar, K. Moeini, A. M. Z. Slawin, and J. D. Woollins. J. Coord. Chem., 2018, 71, 120. https://doi.org/10.1080/00958972.2018.1426852
G. Jones, P. Willett, R. C. Glen, A. R. Leach, and R. Taylor. J. Mol. Biol., 1997, 267, 727-748. https://doi.org/10.1006/jmbi.1996.0897
Drugs.com: Doxorubicin. https://www.drugs.com/mtm/doxorubicin.html (accessed Nov 07, 2018).
A. Usman, H.-K. Fun, S. Chantrapromma, H.-L. Zhu, and Q.-X. Liu. Acta Crystallogr., Sect. E, 2003, 59, o215. https://doi.org/10.1107/S1600536803001144
J.-Y. Ma, B.-L. Lv, S.-H. Gu, J.-W. Guo, and W.-P. Yin. Acta Crystallogr., Sect. E, 2006, 62, m1322. https://doi.org/10.1107/S1600536806017016
T. Chattopadhyay, M. Mukherjee, K. S. Banu, A. Banerjee, E. Suresh, E. Zangrando, and D. Das. J. Coord. Chem., 2009, 62, 967. https://doi.org/10.1080/00958970802385837
S.-J. Peng, H.-Y. Hou, and C.-S. Zhou. Synth. React. Inorg. Nano-Met. Chem., 2009, 39, 462. https://doi.org/10.1080/15533170903228190
N. A. Ikmal Hisham, H. Khaledi, and H. Mohd Ali. Acta Crystallogr., Sect. E, 2011, 67, m932. https://doi.org/10.1107/S1600536811022021
Y. J. Wei, F. W. Wang, and Q. Y. Zhu. Acta Crystallogr., Sect. E, 2008, 64, m859. https://doi.org/10.1107/S1600536808015730
D. F. Zhang, M. H. Zhou, and C. J. Yuan. Acta Crystallogr., Sect. E, 2008, 64, m825. https://doi.org/10.1107/S1600536808014311
X.-Y. Qiu. Acta Crystallogr., Sect. E, 2006, 62, m717. https://doi.org/10.1107/S1600536806007562
X.-F. Li, Z.-L. You, P. Hou, and C.-L. Zhang. J. Chem. Crystallogr., 2010, 40, 561. https://doi.org/10.1007/s10870-010-9696-8
S.-J. Peng, C.-S. Zhou, and T. Yang. Acta Crystallogr., Sect. E, 2006, 62, m1147. https://doi.org/10.1107/S1600536806014759
X. W. Zhu and X. Z. Yang. Acta Crystallogr., Sect. E, 2008, 64, m1090. https://doi.org/10.1107/S1600536808023659
X. W. Zhu. Acta Crystallogr., Sect. E, 2008, 64, m1456. https://doi.org/10.1107/S1600536808033977
S.-S. Qian, H.-H. Li, H. Zhu, Z.-M. Yang, Z.-L. You, and H.-L. Zhu. Synth. React. Inorg. Nano-Met. Chem., 2013, 43, 412. https://doi.org/10.1080/15533174.2012.740742
Y. X. Sun and Z. L. You. Synth. React. Inorg. Nano-Met. Chem., 2007, 36, 359. https://doi.org/10.1002/ejic.200790003
F. Marandi, K. Moeini, and H. Krautscheid. Acta Crystallogr., Sect. C, 2019, 75, 1389. https://doi.org/10.1107/S2053229619011719
J. M. German-Acacio, H. Tlahuext, and H. Hopfl. Acta Crystallogr., Sect. E, 2011, 67, o2849. https://doi.org/10.1107/S1600536811039997
G. Sheldrick. Acta Crystallogr., Sect. A, 2014, 70, C1437. https://doi.org/10.1107/S2053273314085623
G. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112. https://doi.org/10.1107/S0108767307043930
L. J. Farrugia. J. Appl. Crystallogr., 1997, 30, 565. https://doi.org/10.1107/S0021889897003117
M. N. Burnett and C. K. Johnson. Ortep-III, Report ORNL-6895. Oak Ridge, Tennessee: Oak Ridge National Laboratory, 1996.
G. Bergerhof, M. Berndt, and K. Brandenburg. J. Res. Natl. Stand. Technol., 1996, 101, 221-225. https://doi.org/10.6028/jres.101.023
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian09. Wallingford, CT: Gaussian, Inc.: 2009.
J. P. Perdew. Phys. Rev. B, 1986, 33, 8822. https://doi.org/10.1103/PhysRevB.33.8822
A. Gavezzotti. Acc. Chem. Res., 1994, 27, 309. https://doi.org/10.1021/ar00046a004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 2, pp. 233-237.https://doi.org/10.26902/JSC_id88425
Rights and permissions
About this article
Cite this article
Esmaeilzadeh, J., Mardani, Z., Moeini, K. et al. COORDINATION OF AN AMINO ALCOHOLIC SCHIFF BASE LIGAND TOWARD THE ZINC(II) ION: SPECTRAL, STRUCTURAL, THEORETICAL, AND DOCKING STUDIES. J Struct Chem 62 (Suppl 1), S8–S19 (2021). https://doi.org/10.1134/S0022476621130023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621130023