Abstract
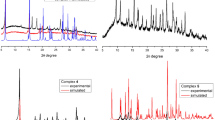
[Cu(L)2] (1), [Co(L)2] (2), [Ni(L)2]·H2O (3) complexes where L is 2-{[(2-methoxyphenyl)amino]methylene}-5,5-dimethyl-cyclohexane-1,3-dionate are synthesized. Single crystals of complexes 1-3 are obtained. According to X-ray crystallographic data, in the Co and Ni complexes, a coordination polyhedron N2O4 is a distorted octahedron, and in the Cu complex, N2O2 is a distorted square. L is a tridentate ligand coordinated by oxygen atoms of carbonyl and methoxy groups as well as the nitrogen atom of the amide group. The cytotoxic properties of 2-{[(2-methoxyphenyl)amino]methylene}-5,5-dimethyl-cyclohexane-1,3-dione and respective complexes are studied on the Hep2 cell line (human laryngeal carcinoma). The compounds are shown to not exhibit cytotoxic properties in the studied concentration range (1-50 μM). Antibacterial, protistocidal, and fungistatic properties of HL, its structural analogue 2-anilinomethylidene-5,5-dimethylcyclohexane-1,3-dione (HL1), and complexes on their base are also studied. The antibacterial activity of HL and [Co(L1)2(C2H5OH)2] against S. aureus, of HL1 and [Co(L)2] against E. coli is revealed within 25-30% of the activity of reference drugs (ciprofloxacin, furazolidone). The [Ni(L1)2(C2H5OH)2] complex is found to have a pronounced protistocidal activity. Furthermore, the behavior of the [Ni(L)2]·H2O complex in phosphate buffered saline is analyzed at storage times t = 0 h, 24 h, 48 h and also in an aqueous solution at different pH values.






Similar content being viewed by others
REFERENCES
Chaaban, J. V. Greenhill, and P. Akhtar. J. Chem. Soc., 1979, 6, 1593, DOI: 10.1039/P19790001593.
J. P. Michael, C. B. De Koning, D. Gravestock, G. D. Hosken, A. S. Howard, C. M. Jungmann, R. W. M. Krause, A. S. Parsons, S. C. Pelly, and T. V. Stanbury. Pure Appl. Chem., 1999, 71, 979, DOI: 10.1351/pac199971060979.
A. C. Spivey, R. Srikaran, C. M. Diaper, and D. J. Turner. Org. Biomol. Chem., 2003, 1, 1638, DOI: 10.1039/B303064D.
F. R. Souza, V. T. Souza, V. Ratzlaff, L. P Borges, M. R. Oliveira, H. G. Bonacorso, N. Zanatta, M. A. P. Martins, and C. F. Mello. Eur. J. Pharmacol., 2002, 451, 141, DOI: 10.1016/S0014-2999(02)02225-2.
J. G. Martinez, P. R. Duchowicz, M. R. Estrada, G. N. Zamarbide, and E. A. Castro. Int. J. Mol. Sci., 2011, 12, 9354, DOI: 10.3390/ijms12129354.
J. P. Michael, C. B. De Koning, G. D. Hosken, and T. V. Stanbury. Tetrahedron, 2001, 57, 9635, DOI: 10.1016/S0040-4020(01)00964-4.
D. L. Boger, T. Ishizaki, R. J. Wysocki, S. A. Munk, P. A. Kitos, and O. Suntornwat. J. Am. Chem. Soc., 1989, 111, 6461, DOI: 10.1021/ja00198a089.
H. M. C. Ferraz and E. R. S. Gonçalo. Quím. Nova, 2007, 30, 957, DOI: 10.1590/S0100-40422007000400035.
V. Bertolasi, L. Pretto, V. Ferretti, P. Gilli, and G. Gilli. Acta Crystallogr., Sect. B, 2006, 62, 1112, DOI: 10.1107/S0108768106036421.
L. Zhang, M. Brookhart, and P. S. White. Organometallics, 2006, 25, 1868, DOI: 10.1021/om050931p.
D. Jones, A. Roberts, K. Cavell, W. Keim, U. Englert, B. W. Skelton, and A. W. White. Dalton Trans., 1998, 255, DOI: 10.1039/A707422K.
S. Mokesch, M. S. Novak, A. Roller, M. A. Jakupec, W. Kandioller, and B. K. Keppler. Organometallics, 2015, 34, 848, DOI: 10.1021/om501032s.
R. Huma, T. Mahmud, L. Mitu, M. Ashraf, A. Iqbal, K. Iftikhar, and A. Hayat. Rev. Chim., 2019, 70, 3564, DOI: 10.37358/RC.19.10.7597.
J. Kim, J.-W. Hwang, Y. Kim, M. H. Lee, and Y. Do. J. Organomet. Chem., 2001, 620, 1, DOI: 10.1016/S0022-328X(00)00555-6.
Y.-C. Shi, W.-B. Shen, H.-M. Yang, H.-B. Song, and X.-Y. Hu. Polyhedron, 2004, 23, 749, DOI: 10.1016/j.poly.2003.10.019.
T. Mahmud, R. Rehman, A. Gulzar, A. Khalid, J. Anwar, U. Shafique, Waheed-uz-Zaman, and M. Salman. Arabian J. Chem., 2010, 3, 219, DOI: 10.1016/j.arabjc.2010.06.003.
B. Jeragh and A.-Z. A. Elassar. Chem. Sci. Trans., 2015, 4, 113, DOI:10.7598/cst2015.965.
R. Huma, T. Mahmud, R. Munir, S. J. Awan, K. Iftikhar, and Mufakkira-Tul-Islam. Pak. J. Zool., 2019, 51, 399, DOI: 10.17582/journal.pjz/2019.51.2.697.702.
S. Mokesch, D. Schwarz, M. Hejl, M. H. M. Klose, A. Roller, M. A. Jakupec, and B. K. Keppler. Molecules, 2019, 24, 2373, DOI:10.3390/molecules24132373.
J. A. Eremina, E. V. Lider, T. S. Sukhikh, I. V. Eltsov, N. V. Kuratieva, B. A. Zakharov, L. A. Sheludyakova, L. S. Klyushova, E. A. Ermakova, and V. V. Dotsenko. Polyhedron, 2020, 178, 114325, DOI: 10.1016/j.poly.2019.114325.
O. S. Wolfbeis and E. Ziegler. Z. Naturforsch., B, 1976, 31, 1519, DOI: 10.1515/znb-1976-1118.
V. V. Dotsenko, S. G. Krivokolysko, A. N. Chernega, and V. P. Litvinov. Russ. Chem. Bull., 2002, 51, 1556, DOI: 10.1023/A:1020939712830.
H.-G. Henning and J.-U. Thurner. Z. Chem., 1978, 18, 256, DOI: 10.1002/zfch.19780180.
Apex3, SADABS-2016/2 and SAINT, version 2018.7-2. Bruker AXS Inc.: Madison, WI, 2017.
CrysAlisPro, 1.171.38.46. Rigaku Oxford Diffraction: The Woodlands, TX, USA, 2015.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2015, 71, 3, DOI: 10.1107/S2053273314026370.
G .M. Sheldrick. Acta Crystallogr., Sect. C, 2015, 71, 3, DOI: 10.1107/S2053229614024218.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339.
J. A. Eremina, E. V. Lider, T. S. Sukhikh, L. S. Klyushova, M. L. Perepechaeva, D. G. Sheven′, A. S. Berezin, A. Y. Grishanova, and V. I. Potkin. Inorg. Chim. Acta, 2020, 510, 119778, DOI: 10.1016/j.ica.2020.119778.
A. S Burlov, Y. V Koshchienko, N. I. Makarova, G. S. Borodkin, A. V. Metelitsa, V. G. Vlasenko, A. A. Zubenko, Y. D. Drobin, Y. V. Zubavichus, and D. A. Garnovskii. Polyhedron, 2018, 144, 249, DOI: 10.1016/j.poly.2018.01.020.
A. S. Burlov, Y. V. Koshchienko, N. I. Makarova, A. A. Kolodina, V. G. Vlasenko, A. A. Zubenko, Y. D. Drobin, L. N. Fetisov, Y. V. Zubavichus, A. L. Trigub, S. I. Levchenkov, and D. A. Garnovskii. Polyhedron, 2018, 154, 65, DOI: 10.1016/j.poly.2018.07.034.
L. N. Fetisov, A. A. Zubenko, A. N. Bodryakov, and M. A. Bodryakova. Vopr. Normativno-Pravovogo Regul. Vet., 2012, 4(1), 70.
Metody Eksperimentalnoy Khimioterapii (Experimental Chemotherapy Methods) [in Ruissian] / Ed. G. N. Pershin. Meditsyna: Moscow, 1971.
S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell, and D. Avnir. Coord. Chem. Rev., 2005, 249, 1693, DOI: 10.1016/j.ccr.2005.03.031.
SHAPE 2.1: Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools. http://www.ee.ub.edu/
M. C. Vlasiou and K. S. Pafiti. Open Med. Chem. J., 2020, 14, 1. DOI: 10.2174/1874104502014010001.
Funding
The work was performed within the State Contract of the Nikolaev Institute of Inorganic Chemistry, Siberian Branch, Russian Academy of Sciences for fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Translated from Zhurnal Strukturnoi Khimii, 2021, Vol. 62, No. 2, pp. 325-337 https://doi.org/10.26902/JSC_id68376 .
Supplementary material
Rights and permissions
About this article
Cite this article
Eremina, Y.A., Ermakova, E.A., Sukhikh, T.S. et al. SYNTHESIS, STRUCTURE, AND STUDY OF THE BIOLOGICAL ACTIVITY OF Co(II), Ni(II), AND Cu(II) COMPLEXES WITH AN ENAMINEDIONE DERIVATIVE. J Struct Chem 62, 309–320 (2021). https://doi.org/10.1134/S0022476621020165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476621020165