Abstract
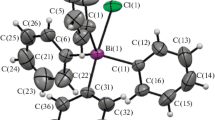
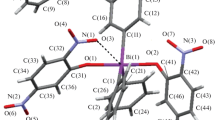
Tetraphenylbismuth hydrosulfate Ph4Bi(OSO2OH) (1), tetraphenylbismuth 2,4-dinitrobenzenesulfonate Ph4Bi(OSO2C6H3(NO2)2-2,4) (2), and tetraphenylbismuth nitrate hydrate Ph4BiNO3·1/3H2O (3) are synthesized by the interaction of pentaphenylbismuth with equimolar amounts of sulfuric, 2,4-dinitrobenzenesulfonic, or nitric acids. The crystal structures of 1–3 are determined by the single crystal X-ray diffraction analysis. In 1 and 2, bismuth atoms are pentacoordinated (C4BiO environment). The 3 crystal contains three types of tetraphenylbismuthonium cations, two of which being coordinated to a nitrate anion and a water molecule.
Similar content being viewed by others
References
Y. Matano, T. Suzuki, T. Shinokura, and H. Imahori. Tetrahedron Lett., 2007, 48, 2885.
Y. Matano, T. Suzuki, T. Iwata, T. Shinokura, and H. Imahori. Bull. Chem. Soc. Jpn., 2008, 81, 1621.
D. H. R. Barton, B. Charpiot, E. T. H. Dau, W. B. Motherwell, C. Pascard, and C. Pichon. Helv. Chim. Acta, 1984, 67, 586.
V. V. Sharutin, I. V. Egorova, O. K. Sharutina, O. A. Dorofeeva, T. K. Ivanenko, A. V. Gerasimenko, and M. A. Pushilin. Russ. J. Coord. Chem., 2004, 30, 874.
V. V. Sharutin, I. V. Egorova, A. P. Pakusina, O. K. Sharutina, and M. A. Pushilin. Russ. J. Coord. Chem., 2007, 33, 168.
V. V. Sharutin, I. V. Egorova, N. N. Klepikov, E. A. Boyarkina, and O. K. Sharutina. Russ. J. Inorg. Chem., 2009, 54, 1768.
V. V. Sharutin, I. V. Egorova, N. N. Klepikov, E. A. Boyarkina, and O. K. Sharutina. Russ. J. Inorg. Chem., 2009, 54, 52.
V. V. Sharutin, O. K. Sharutina, I. V. Egorova, A. N. Kharsika, O. A. Lodochnikova, A. T. Gubaidullin, and I. A. Litvinov. Russ. Chem. Bull., 1999, 48, 2325.
V. V. Sharutin, O. K. Sharutina, I. V. Egorova, V. S. Senchurin, A. N. Zakharova, and V. K. Bel’skii. Russ. J. Gen. Chem., 1999, 69, 1414.
S. Hoppe and K. H. Whitmire. Organometallics, 1998, 17, 1347.
V. V. Sharutin, I. V. Egorova, T. V. Tsiplukhina, A. V. Gerasimenko, and M. A. Pushilin. Russ. J. Coord. Chem., 2004, 30, 884.
V. V. Sharutin, I. V. Egorova, O. K. Sharutina, A. P. Pakusina, and M. A. Pushilin. Russ. J. Coord. Chem., 2008, 34, 85.
V. V. Sharutin, I. V. Egorova, T. K. Ivanenko, O. K. Sharutina, I. I. Pavlushkina, and A. V. Gerasimenko. Russ. J. Coord. Chem., 2003, 29, 317.
V. V. Sharutin, I. V. Egorova, T. K. Ivanenko, O. K. Sharutina, and D. Yu. Popov. Russ. J. Coord. Chem., 2003, 29, 468.
T. Ooi, R. Goto, and K. Maruoka. J. Am. Chem. Soc., 2003, 125, 10494.
K. A. Kocheshkov, A. P. Skoldinov, and N. N. Zemlyansky. Metody elementoorganicheskoj himii. Sur’ma, vismut (Methods of the Organometallic Chemistry. Antimony, Bismuth) [in Rissian]. Nauka: Moscow, 1976.
Bruker. SMART and SAINT-Plus. Versions 5.0. Data Collection and Processing Software for the SMART System. Madison, Wisconsin, Bruker AXS Inc.: USA, 1998.
Bruker. SHELXTL/PC. Versions 5.10. An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data. Madison, Wisconsin, Bruker AXS Inc.: USA, 1998.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. J. Appl. Crystallogr., 2009, 42, 339.
R. Rüther, F. Huber, and H. Preut. Z. Anorg. Allg. Chem., 1986, 539, 110.
M. Mantina, A. C. Chamberlin, R. Valero, C. J. Cramer, and D. G. Truhlar. J. Phys. Chem. A, 2009, 113, 5806.
B. N. Tarasevich. IK Spektry Osnovnyh Klassov Organicheskih Soedinenij (IR Spectra of the Main Classes of Organic Compounds). MGU: Moscow, 2012.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interests.
Additional information
Russian Text © The Author(s), 2020, published in Zhurnal Strukturnoi Khimii, 2020, Vol. 61, No. 5, pp. 776–783.
Rights and permissions
About this article
Cite this article
Sharutin, V.V., Sharutina, O.K. & Senchurin, V.S. Study of the Crystal Structures of Tetraphenylbismuth Hydrosulfate, Tetraphenylbismuth 2,4-Dinitrobenzenesulfonate, and an Adduct of Tetraphenylbismuth Nitrate with Water. J Struct Chem 61, 734–741 (2020). https://doi.org/10.1134/S0022476620050091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476620050091