Abstract
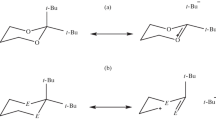
In this study, the stability of 2-methyl-1,3,2-diheterophosphinane 2-oxide conformers (heteroatom = O, S, Se) is investigated at the B3LYP/6-311+G(d,p) level of theory. The total energy, dipole moment, the energies of frontier orbitals, and HOMO-LUMO gaps of these molecules are calculated. The NBO analysis is applied to illustrate the hyperconjugative anomeric effect on the conformers. The responsible interactions of this effect are determined. The interaction energy, off-diagonal elements, and the total steric exchange energy (TSEE) of these interactions and Wiberg indices of bond values are estimated. Also, changes in the calculated bond distances are explained based on the natural bond orbital (NBO) analysis.
Similar content being viewed by others
References
H. G. Khorana. Some Recent Developments in the Chemistry of Phosphate Esters of Biological Interest. John Wiley and Sons, Inc., New York, 1961.
R. S. Edmundson. Chem. Ind., 1967, 1809.
N. A. Loshadkin, V. V. Smirnov. A Review of Modern Literature on the Chemistry and Toxicology of Organophosphorus Inhibitors of Cholinesterases (Translated from Russian by G. M. Kosolapoff). Associated Technical Services, Inc., Glen Ridge, New Jersey, 1962.
R. D. O’Brien. Toxic Phosphorus Esters: Chemistry, Metabolism and Biological Effects. Academic Press: New York, 1960.
B. C. Saunders. Some Aspects of the Chemistry and Toxic Action of Organic Compounds Containing Phosphorus and Fluorine. Cambridge University Press: Cambridge, England, 1967.
E. L. Eliel, S. H. Wilen, and L. N. Mander. Stereochemistry of Organic Compounds. Wiley: New York, 1994.
G. M. Kellie and F. G. Ridell. Top. Stereochem., 1974, 8, 225.
E. L. Eliel and M. C. Knoeber. J. Am. Chem. Soc., 1968, 90, 3444.
F. G. Ridell and J. T. Robinson. Tetrahedron, 1967, 23, 3417.
K. Pihlaja. Acta Chem. Scand., 1948, 22, 716.
W. G. Bentrude. In: Analysis, Dynamics and Stereoelectronic Effects / Ed. E. Juaristi. New York: VCH, 1995.
B. E. Maryanoff, R. O. Hutchins, and C. A. Maryanoff. Top. Stereochem., 1979, 11, 187.
D. G. Gorenstein. Chem. Rev., 1987, 87, 1049.
W. G. Bentrude and H.-W. Tan. J. Am. Chem. Soc., 1973, 95, 4666.
P. V. Nuffel, C. V. Alsenoy, A. T. H. Lenstra, and H. J. Geise. J. Mol. Struct., 1984, 125, 1.
J. P. Majoral, C. Bergounhou, and J. Navech. J. Bull. Soc. Chim. Fr., 1973, 3146.
J.-F. Brault, J. P. Majoral, P. Savignac, and J. Navech. J. Bull. Soc. Chim. Fr., 1973, 3149.
G. R. J. Thatcher. The Anomeric Effect and Associated Stereoelectronic Effects. American Chemical Society, Washington, DC, 1992, 539.
E. Juaristi and G. Cuevas. Tetrahedron, 1992, 48, 5019.
A. J. Kirby. The Anomeric Effect and Related Stereoelectronic Effects at Oxygen. Springer: Heidelberg, Germany, 1983.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalman, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox. Gaussian 09 (Version Revision A.02). Wallingford CT: Gaussian, Inc, 2009.
R. Krishnan, J. S. Binkley, R. Seeger, and J. A. Pople. J. Chem. Phys., 1980, 72, 650.
A. D. McLean and G. S. Chandler. J. Chem. Phys., 1980, 72, 5639.
L. A. Curtiss, M. P. McGrath, J.-P. Blandeau, N. E. Davis, R. C. Binning, and J. L. Radom. J. Chem. Phys., 1995, 103, 6104.
A. D. Becke. J. Chem. Phys, 1993, 98, 5648.
A. E. Reed, L. A. Curtiss, and F. Weinhold. Chem. Rev., 1988, 88, 899.
E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales, C. R. Landis, and F. Weinhold. NBO 6.0. Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2013.
T. Lu and F. Chen. J. Comput. Chem., 2012, 33, 580.
C. L. Perrin, K. B. Armstrong, and M. A. Fabian. J. Am. Chem. Soc., 1994, 116, 715.
C. James, A. Amal Raj, R. Reghunathan, I. H. Joe, and V. S. Jayakumar. J. Raman Spectrosc., 2006, 37, 1381.
L. J. Na, Z. Rang, and Y. S. Fang. J. Zhejiang Univ. Sci., 2005, 6B, 584.
Y. Yang, W. J. Zhang, and X. M. Gao. Int. J. Quantum Chem., 2006, 106, 1199.
J.-P. Praly and R. U. Lemieux. Can. J. Chem., 1987, 213.
P. A. Christiansen and W. E. Palke. J. Chem. Phys., 1977, 67, 57.
V. F. Weisskopf. Science, 1975, 187, 605.
J. K. Badenhoop and F. Weinhold. Int. J. Quantum. Chem., 1999, 72, 269.
Author information
Authors and Affiliations
Corresponding author
Additional information
Text © The Author(s), 2019, published in Zhurnal Strukturnoi Khimii, 2019, Vol. 60, No. 5, pp. 779-787.
Rights and permissions
About this article
Cite this article
Nasrolahi, M., Ghiasi, R. & Shafiee, F. Stability, Electronic, and Structural Features of the Conformers of 2-Methyl-1,3,2-Diheterophosphinane 2-Oxide (Heteroatom = O, S, Se): DFT and NBO Investigations. J Struct Chem 60, 746–754 (2019). https://doi.org/10.1134/S0022476619050068
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476619050068