Abstract
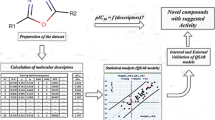
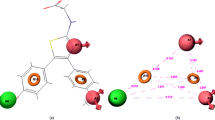
A three-dimensional quantitative structure-activity relationship (3D-QSAR) model of 45 sulfonylurea herbicidals is established using the Topomer comparative molecular field analysis (Topomer CoMFA). The results show that correlation coefficient (q2), non-cross-validation correlation coefficient (r2), and external validation (Q 2ext ) are 0.884, 0.908, and 0.857 respectively. The biological evaluation demonstrates that the obtained model has the excellent external predictive ability and good estimation stability. The methodology of the fragment-based drug design is also applied to design new sulfonylurea herbicides using the Topomer Search technology. Compound 11 with the highest activity is chosen as the template molecule. By adding three substitutional groups selected from the ZINC database to the template molecule, 36 new compounds with an activity more than that of the template molecule are obtained. The template molecular and designed compounds are used to explore the binding relationship of the ligands with the receptor protein in molecular docking. The result shows that hydrogen bonding interactions form between the ligands and amino acid residues.
Similar content being viewed by others
References
D. Saja, M. Rys, I. Stawoska, and A. Skoczowski. Acta Physiol Plant., 2016, 38(7), 1.
B. L. Wang, Y. X. Shi, S. J. Zhang, and Y. Ma. Eur. J. Med. Chem., 2016, 117(19), 167.
G. Levitt. ACS Symp. Ser., 1991, 443, 6.
M. H. Dong, H. F. Chen, and Y. J. Ren. Bioorg. Med. Chem., 2015, 24(2), 73.
K. Roy, S. Kar, and P. Ambure. Chemom. Intell. Lab. Syst., 2015, 145, 22.
R. D. Cramer, P. Cruz, and G. Stahl. J. Chem. Inf. Mode., 2008, 48(11), 2180.
R. J. Jilek and R. D. Cramer. J. Chem. Inf. Comput. Sci., 2004, 44(4), 1221.
R. M. Asath, T. N. Rekha, S. Premkumar, T. Mathavan, et al. J. Mol. Struct., 2016, 1125(5), 633.
J. A. Mccourt, S. S. Pang, L. W. Guddat, and R. G. Duggleby. Biochemistry US., 2005, 44(7), 238.
K. Roy and S. Paul. J. Mol. Model., 2010, 6(5), 951.
W. Wei, D. D. Cheng, J. B. Liu, and Y. X. Li. Org. Biomol. Chem., 2016, 14(35), 8356.
J. G. Wang, P. K. Lee, and Y. H. Dong. FEBS J., 2009, 276(5), 1282.
C. Vats, J. K. Dhanjal, S. Goyal, N. Bharadvaja, and A. Grover. Struct. Chem., 2015, 26, 467.
C. Xu and Y. J. Ren. Bioorg. Med. Chem. Lett., 2015, 25(20), 4522.
G. Tresaderna, J. M. Cidb, and A. A. Trabanco. J. Mol. Graph. Model., 2014, 53, 82.
G. Subramanian and S. N. Rao. Bioorg. Med. Chem., 2012, 20(20), 851.
D. D. Huang, Y. L. Liu, B. Z. Shi, Y. T. Li, G. X. Wang, and G. Z. Liang. J. Mol. Graph Model., 2013, 45(18), 65.
H. Zhi, J. X. Zheng, and Y. Q. Chang. J. Mol. Struct., 2015, 1098(5), 199.
A. Singh, S. Goyal, S. Jamal, and B. Subramani. Struct. Chem., 2016, 27(3), 993.
Y. Y. Lv, C. S. Yin, and H. Y. Liu. J. Environ Sci., 2008, 20(12), 1433.
X. Y. Wu and W. H. Chen. Int J. Mol. Sci., 2012, 13(2), 2387.
J. B. Bhonsle, V. Divakaramenon, and D. P. Huddler. J. Med. Chem., 2008, 50(26), 6545.
P. P. Roy, J. T. Leonard, and K. Roy. Intell. Lab., 2008, 90(1), 31.
Y. H. Xiang, J. Song, and Z. Y. Zhang. Comb. Chem. High Throughput Screening, 2014, 17(5), 45.
J. B. Tong, M. Bai, and X. Zhao. Med. Chem. Res., 2016, 25(11), 2619.
M. M. Ghorab, F. A. Ragab, and H. I. Heiba. Eur. J. Med. Chem., 2016, 117(19), 8.
X. Q. Yan, Z. C. Wang, Z. Li, and P. F. Wang. Med. Chem. Lett., 2015, 25(20), 4664.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhurnal Strukturnoi Khimii, Vol. 60, No. 2, pp. 222–230, February, 2019.
Electronic supplementary material
10947_2019_1126_MOESM1_ESM.pdf
Molecular Virtual Screening Studies of Herbicidal Sulfonylurea Analogues Using Molecular Docking and Topomer CoMFA Research
Rights and permissions
About this article
Cite this article
Tong, J., Jiang, G., Li, L. et al. Molecular Virtual Screening Studies of Herbicidal Sulfonylurea Analogues Using Molecular Docking and Topomer CoMFA Research. J Struct Chem 60, 210–218 (2019). https://doi.org/10.1134/S0022476619020057
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476619020057