Abstract
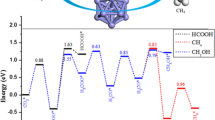
The preferential oxidation (PROX, CO + H2 + O2 → CO2 + H2O) of the CO reaction in an H2 stream is the simplest and most cost-effective method to remove CO gas to less than 10 ppm in reformed fuel gas. We study the mechanism of PROX of the CO reaction in the H2 stream catalyzed by Cu n Ni (n = 3-12) clusters using a density functional theory (DFT) calculation to investigate bimetallic effects on the catalytic activation. Our results indicate that the Cu12Ni cluster is the most efficient catalyst for H2 dissociation and the Cu6Ni cluster is the most efficient catalyst for CO-PROX in excess hydrogen among Cu n Ni (n = 3-12) clusters. To gain insight into the adsorption and dissociation of the H2 molecule effect in the catalytic activity over the Cu12Ni cluster and the potential energy surfaces about PROX of CO oxidation on the Cu6Ni cluster, the nature of the interaction between the adsorbate and substrate is analyzed by detailed electron local densities of states (LDOS) as well as molecular structures.
Similar content being viewed by others
References
C. E. Carlton, S. Chen, P. J. Ferreira, L. F. Allard, and Y. S. Horn, J. Phys. Chem. Lett., 3, 161–166 (2012).
H. Wakita, K. Ukai, T. Takeguchi, and W. Ueda, J. Phys. Chem. C, 111, 2205–2211 (2007).
K. C. Lauzze and D. J. Chmielewski, Ind. Eng. Chem. Res. 45, 4661–4670 (2006).
K. Liu, A. Wang, and T. Zhang, ACS Catal., 2, 1165–1178 (2012).
J. G. E. Cohn, Patented Nov., 9, 3216782 (1965).
J. Gustafson, R. Westerstro, O. Balmes, A. Resta, R. Rijn, X. Torrelles, C. T. Herbschleb, J. W. M. Frenken, and E. Lundgren, J. Phys. Chem. C, 114, 4580–4583 (2010).
T. Tabakova, M. Manzoli, F. Vindigni, V. Idakiev, and F. Boccuzzi, J. Phys. Chem. A, 114, 3909–3915 (2010).
O. H. Laguna, W. Y. Hernandez, G. Arzamendi, L. M. Gandia, M. A. Centeno, and J. A. Odriozola, Fuel 118, 176–185 (2014).
P. Sangeetha, L. H. Chang, and Y. W. Chen, Ind. Eng. Chem. Res. 48, 5666–5670 (2009).
A. Fukuoka, J. Kimura, T. Oshio, Y. Sakamoto, and M. Ichikawa, J. Am. Chem. Soc., 129, 10120–10125 (2007).
H. Zhang, M. Jin, H. Liu, J. Wang, M. J. Kim, D. Yang, Z. Xie, J. Liu, and Y. Xia, ACSNano, 5, 8212–8222 (2011).
E. Y. Ko, E. D. Park, H. C. Lee, D. Lee, and S. Kim, Angew. Chem. Int. Ed. 46, 734–737 (2007).
Z. Y. Pu, X. S. Liu, A. P. Jia, Y. L. Xie, J. Q. Lu, and M. F. Luo, J. Phys. Chem. C, 112, 15045–15051 (2008).
B. Yang, R. Burch, G. Hardacre, and Hu. P. Headdock, ACS Catal. 2, 1027–1032 (2012).
W. Wei, Y. Dai, and B. Huang, J. Phys. Chem. C, 115, 18597–18602 (2011).
V. Umamaheswari, M. Hartmann, and A. Pöppl, J. Phys. Chem. B, 109, 1537–1546 (2005).
S. Shen, L. Zhao, Z. Zhou, and L. Guo, J. Phys. Chem. C, 112, 16148–16155 (2008).
A. M. Arias, D. Gamarra, M. F. García, A. Hornés, P. Bera, Z. Koppány, and Z. Schay, Catal. Today 143, 211–217 (2009).
X. Liua, A. Wanga, T. Zhanga, D. Sub, and C. Y. Mouc, Catal. Today 160, 103–108 (2011).
T. Komatsu, M. Takasaki, K. Ozawa, S. Furukawa, and A. Muramatsun, J. Phys. Chem. C, 117, 10483–10491 (2013).
J. Kugai, T. Moriya, S. Seino, T. Nakagawa, Y. Ohkubo, H. Nitani, K. Ueno, and T. A. Yamamoto, J. Phys. Chem. C, 117, 5742–5751 (2013).
L. Y. Gan, R. Y. Tian, X. B. Yang, H. D. Lu, and Y. J. Zhao, J. Phys. Chem. C, 116, 745–752 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Jr. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Hey, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision A.1, Gaussian, Inc. Wallingford CT. (2009).
J. P. Perdew, K. Burke, and M. Ernzerhof, Phys. Rev. Lett. 77, 3865–3868 (1996).
W. R. Wadt and P. J. Hay, J. Chem. Phys., 82, 270–283 (1985).
P. J. Hay and W. R. Wadt, J. Chem. Phys., 82, 299–310 (1985).
C. Peng, P. Y. Ayala, and H. B. Schlegel, J. Comput. Chem., 17, 49–56 (1996)
C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 90, 2154–2161 (1989)
C. Gonzalez and H. B. Schlegel, J. Chem. Phys., 54, 5523–5527 (1990).
E. D. Glendening, A. E. Reed, and J. E. Carpenter, Weinhold. NBO, Theoretical Chemistry Institute, University of Wisconsin Madison (2001).
K. P. Huber and G. Herzberg, Constants of Diatomic Molecules, Van Nostrand Reinhold, New York (1979).
M. D. Morse, Chem. Rev. 86, 1049–1109 (1988).
B. Yin, Y. Yin, Y. Lei, L. Dong, and Y. Zhang, Chem. Phys. Lett. 509, 192–197 (2011).
Z. Cao, Y. Wang, J. Zhu, W. Wu, and Q. Zhang, J. Phys. Chem. B, 106, 9649–9654 (2002).
M. Yang, F. Yang, K. A. Jackson, and J. Jellinek, J. Chem. Phys., 132, 064306 (2010).
E. Florez, F. Mondragón, and P. Fuentealba, J. Phys. Chem. B, 110, 13793–13798 (2006).
S. L. Han, X. Xue, X. C. Nie, H. Zhai, F. Wang, Q. Sun, Y. Jia, S. F. Li, and Z. X. Guo, Phys. Lett. A 374, 4324–4330 (2010).
K. M. Tanaka and Y. Shou, J. Phys. Chem. C, 114, 16917–16923 (2010).
K. Tanaka, M. Shou, H. He, X. Shi, and X. Zhang, J. Phys. Chem. C, 113, 12427–12433 (2009).
A. W. Pelzer, J. Jellinek, and K. A. Jackson, J. Phys. Chem. A, 117, 10407–10415 (2013).
Q. Fu and Y. Luo, J. Phys. Chem. C, 117, 14618–14624 (2013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2017 N. Liu, L. Guo, C. Wen, Z. Cao.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 58, No. 8, pp. 1661-1674, November-December, 2017.
Rights and permissions
About this article
Cite this article
Liu, N., Guo, L., Wen, C. et al. Reaction mechanism of the preferential oxidation of the CO reaction in an H2 stream over Cu–Ni bimetallic catalysts: A computational study. J Struct Chem 58, 1611–1624 (2017). https://doi.org/10.1134/S0022476617080194
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617080194