Abstract
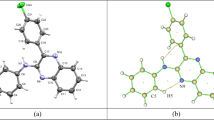
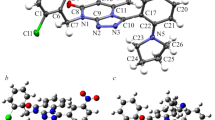
Nonsteroidal anti-inflammatory drugs (NSAIDs) are among the most frequently prescribed drugs and have multiple therapeutic uses. These drugs are predominantly used for the treatment of musculoskeletal diseases because of their analgesic, antipyretic, and antiplatelet activities. Oxicams constitute an interesting class of organic compounds and have been investigated in the search for new analgesic and anti-inflammatory drugs. In the present work, a theoretical investigation of the molecular structure and spectroscopic properties of a series of five oxicams in different solvents was performed using density functional theory (DFT) methods. The geometric optimizations of the oxicams were carried out using the M06 density functional and the CBSB7 basis set. The infrared data were all obtained at the same theoretical level. The UV-Vis absorption and NMR data of some oxicams were calculated using the DFT and CBSB3 basis sets. The analysis of structural parameters, particularly the bond length and spectroscopic data, indicated that interactions occurred between the hydrogen bond types for 4-meloxicam, isoxicam, and normeloxicam. Stereoelectronic interactions caused by the substitution of alkyl groups caused the bond lengths to elongate. Similarly, the substitution of heteroatoms, such as nitrogen, sulfur, or oxygen, increased the bond lengths and angular stresses.
Similar content being viewed by others
References
B. Cronstein and G. Weissmann, Annu. Rev. Pharmacol. Toxicol., 35, 449 (1995).
E. Lazer, C. Miao, C. Cywin, R. Sorcek, H. Wong, Z. Meng, I. Potocki, M. Hoermann, R. Snow, M. Tschantz, T. Kelly, D. McNeil, S. Coutts, L. Churchill, A. Graham, E. David, P. Grob, W. Engel, H. Meier, and G. Trummlitz, J. Med. Chem., 40, 980 (1997).
M. Bianchi and A. Panerai, Pharmacol. Res., 45, 101 (2002).
J. Dogn′e, C. Supur′an, and D. Pratico, J. Med. Chem., 48, 2251 (2005).
J. Ho, M. L. Coote, M. Franco-Perez, and R. Gomez-Balderas, J. Phys. Chem. A, 11992, 114 (2010).
M. Lúcio, H. Ferreira, J. L. F. C. Lima, and S. Reis, Med. Chem., 447, 2 (2006).
J. Martínez-Araya, G. Salgado-Moran, and D. Glossman-Mitnik, J. Phys. Chem. B, 6339, 117 (2013).
G. Salgado-Moran, L. Gerli-Candia, J. Martinez-Araya, R. Ramirez-Tagle, and D. Glossman-Mitnik, Int. J. Pharm. Bio. Sci., 374, 4 (2013).
A. Ghaempanah, S. Jameh-Bozorghi, M. Darvishpour, and M. H. Fekri, Int. J. Electrochem. Sci., 6127, 7 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, H. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Bu-rant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, A. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision A.1., Program for Quantum Chemical Calculations, Gaussian Inc., Wallingford, CT (2009).
Y. Zhao and D. G. Truhlar, Acc. Chem. Res., 41, 157 (2008).
Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 120, 215 (2008).
Y. Zhao and D. G. Truhlar, Chem. Phys. Lett., 502, 1 (2011).
J. Montgomery, M. Frisch, J. Ochterski, and G. Petersson, J. Chem. Phys., 110, 2822 (1999).
J. Montgomery, M. Frisch, J. Ochterski, and G. Petersson, J. Chem. Phys., 112, 6532 (2000).
J. Tomasi, B. Mennucci, and E. Cances, J. Mol. Struct.: THEOCHEM, 464, 211 (1999).
V. N. Emel′yanenko, S. P. Verevkin, E. N. Burakova, G. N. Roganov, and M. K. Georgieva, Russ. J. Phys. Chem. A, 83, 697 (2009).
E. Lewars, Computational Chemistry–Introduction to the Theory and Applications of Molecular and Quantum Mechanics, Kluwer Academic Publishers, Dordrecht (2003).
R. Stratmann, G. Scuseria, and M. Frisch, J. Chem. Phys., 109, 8218 (1998).
R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 256, 454 (1996).
M. E. Casida, C. Jamorski, K. C. Casida, and D. R. Salahub, J. Chem. Phys., 108, 4439 (1998).
Swizard Program Revision 4.6. Program for Postprocessing of Spectral Data, University of Ottawa, Canada (2010).
S. Gorelsky and A. Lever, J. Organomet. Chem., 635, 187 (2001).
A. Allouche, J. Comput. Chem., 32, 174 (2011).
A. Ghaenpanah, S. Jameh-Bozorghi, M. Darvisshpour, and M. Fekri, Int. J. Electrochem. Sci., 7, 6127 (2012).
R. Banerjee, H. Chakraborty, and M. Sarkar, Spectrochim. Acta, Part A, 59, 1213 (2003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2017 A. G. Pacheco, G. Salgado-Morán, L. Gerli-Candia, R. Ramírez-Tagle, D. Glossman-Mitnik, A. Misra, A. F. de Carvalho Alcântara.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 58, No. 2, pp. 278–284, February–March, 2017.
Rights and permissions
About this article
Cite this article
Pacheco, A.G., Salgado-Morán, G., Gerli-Candia, L. et al. Theoretical investigation of the molecular structure and spectroscopic properties of oxicams. J Struct Chem 58, 261–267 (2017). https://doi.org/10.1134/S0022476617020068
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476617020068