Abstract
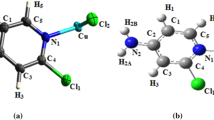
The crystal structure of dichlorobis(dimethylsulfoxide-O)copper(II), [CuCl2(DMSO)2] (I), previously determined by Willett and Chang, is reinvestigated. It crystallizes in the orthorhombic system with the space group Pnma (N°62), Z = 4, and unit cell parameters a = 8.053(1) Å, b = 11.642(5) Å, c = 11.347(3) Å. Our structure determination is of a significantly higher precision in terms of bond lengths, angles, and R factors (e.g., Cu1–O1 = 1.9737(24) Å, O1–Cu1–O1i = 173.08(13)° (symmetry code: I x, 1/2–y, z) and R(F 2) = 0.046 compared to 1.955(4) Å, 173.0(3)° and R(F) = 0.075). In contrast to the previous investigation, all H atoms are placed at calculated positions. In the title molecule, the CuII atom is five coordinated in a distorted square pyramidal geometry. Thus, as reported previously, it can be shown that the crystal structure consists of [CuCl2(DMSO)2] molecules which, by virtue of long Cu–Cl interactions, are tied together to form chains parallel to the [100] direction. The density functional theory (DFT) optimized structure at the B3LYP/6-311++G(2d,2p) level is compared with the experimentally determined molecular structure. The HOMO-LUMO energy gap and other related molecular properties are also calculated. Comprehensive experimental and theoretical structural studies on the studied complex are carried out by FT-IR and UV-visible spectroscopies.
Similar content being viewed by others
References
F. A. Cotton and R. Francis, J. Am. Chem. Soc., 82, 2986–2991 (1960).
F. A. Cotton, R. Francis, and W. D. Horrocks, J. Phys. Chem., 64, 1534–1536 (1960).
F. A. Cotton and R. Francis, J. Inorg. Nucl. Chem., 17, 62–68 (1961).
D. W. Meek, D. K. Straub, and R. S. Drago, J. Am. Chem. Soc., 82, 6013–6016 (1960).
R. S. Drago and D. W. Meek, J. Phys. Chem., 65, 1446 (1961).
A. C. T. North, D. C. Phillips, and F. S. Mathews, Acta Crystallogr., A24, 351–359 (1968).
K. Harms and S. Wocadlo, XCAD4, University of Marburg, Germany (1995).
L. J. Farrugia, J. Appl. Crystallogr., 45, 849–854 (2012).
G. M. Sheldrick, SHELXS-97, SHELXL-97, Acta Crystallogr., A64, 112–122 (2008).
K. Brandenburg, DIAMOND, Crystal Impact GbR, Bonn, Germany (2001).
P. Hohenberg and W. Kohn, Phys. Rev., B136, 864 (1964).
W. Kohn, Phys. Rev., 133, 171–181 (1964).
A. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. Parr, Phys. Rev., B37, 785–789 (1988).
C. W. Bauschlicher and H. Partridge, Chem. Phys. Lett., 240, 533–540 (1995).
M. J. Frisch et al., GAUSSIAN09. Revision A.01, Gaussian Inc., Wallingford, CT, USA (2009).
R. D. Dennington, T. A. Keith, and J. M. Millam, GaussView 5.0.8, Gaussian Inc. (2008).
H. Gokce and S. Bahceli, Spectrochim. Acta, A: Mol. Biomol. Spectrosc., 116, 242–250 (2013).
N. Sundaraganesan, B. D. Joshua, and K. Settu, Spectrochim. Acta, A: Mol. Biomol. Spectrosc., 66, 381–388 (2007).
S. Adams, SoftBV program, University of Göttingen, Germany (2003); http: // kristall.unimki.dwdg.de/softBV.
B. Smith, Infrared Spectral, a Systematic Approach, CRC Press, Washington, DC (1999).
M. Gussoni and C. O. Castiglioni, J. Mol. Struct., 521, 1–8 (2000).
P. S. Kalsi, Spectroscopy of Organic Compounds, New Age International (P) (2009).
L. S. Mohammed, I. S. Hamza, F. R. Muhi AL-Deen, and B. R. J. Muhyedeen, J. Appl. Chem., 3, No. 5, 2102–2121 (2014).
B. Kosar and C. Albayrak, Spectrochim. Acta, 78A, 160–167 (2011).
B. J. Powell, T. Baruah, N. Bernstein, K. Brake, R. H. McKenzie, P. Meredith, and M. R. Pederson, J. Chem. Phys., 12, 8608–8615 (2004).
R. Parr, L. Szentpaly, and S. Liu, Am. Chem. Soc., 121, 1922–1924 (1999).
P. Chattraj, B. Maiti, and U. Sarkar, J. Phys. Chem., A107, 4973–4975 (2003).
T. A. Koopmans, Physica, 1, 104–113 (1934).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2016 H. Chebbi, M. Chebbi, A. Guesmi, Y. Arfaoui.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 57, No. 6, pp. 1164-1170, July-August, 2016.
Rights and permissions
About this article
Cite this article
Chebbi, H., Chebbi, M., Guesmi, A. et al. Crystal structure determination, and DFT Calculations of dichlorobis-(dimethylsulfoxide-O)copper(II). J Struct Chem 57, 1104–1110 (2016). https://doi.org/10.1134/S002247661606007X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002247661606007X