Abstract
Although the experience of optogenetic retinal prosthetics in animal models dates back to more than 16 years, the first results obtained on humans have only been reported in the last year. Over this period, the main challenges of prosthetics became clear and the approaches to their solution were proposed. In this review, we aim to present the achievements in the field of optogenetic prosthetization of retinal bipolar cells with a focus mainly on relatively recent publications. The review addresses the advantages and disadvantages of bipolar cell prosthetics as compared to the alternative target, retinal ganglion cells, and provides a comparative analysis of the effectiveness of ionotropic light-sensitive proteins (channelrhodopsins) or metabotropic receptors (rhodopsins) as prosthetic tools.
Similar content being viewed by others
INTRODUCTION
In recent years, we have witnessed the accelerated development of prosthetic technologies aimed to replace, at least in part, one or another sensory function, primarily vision and hearing, that have been lost completely or to a large extent. For the time being, the most elaborate and effective approach to vision prosthetics is the implantation of an electronic chip that replaces dead sensory cells and provides sensory input for retinal neurons that have survived pathological processes. More than 400 surgical implantations of various prosthetic chips worldwide have been largely successful, having provided a partial vision restoration and allowing patients to distinguish large objects, orient in space, and, in most successful cases, perform such complex actions as archery and bicycling [1–3]. The technological development of electronic chips has been successfully going on for more than 15 years, although it is already clear that the main intractable challenges of this approach are a tendency of glial cells to steadily overgrow the chip contact area, which renders it impossible to use this approach for longer than several years, as well as a very high individual cost of the prosthetic procedure [3].
Optogenetic retinal prosthetics is an alternative to electronic implants. This novel approach becomes possible due to the fact that neurodegenerative processes mainly and primarily affect the photoreceptor cell layer, leaving bipolar and ganglion cells relatively undamaged. From this, it follows that the main idea of the optogenetic approach to retinal prosthetics consists in imparting a property of light sensitivity to bipolar or ganglion cells via genetic manipulations. By now, while the experience of optogenetic retinal prosthetics on animal models dates back to more than 16 years, the first results obtained on humans have only been reported in the last year. During this period, the main challenges of prosthetics have become clear and the approaches to their solution have been proposed (the data are summarized in [4–8]). In this review, we aim to present the most recent achievements in the field of optogenetic bipolar cell prosthetics. We will focus on the advantages and disadvantages of bipolar cell prosthetics as compared to the alternative target, ganglion cells, as well as provide a comparative analysis of the effectiveness of using ionotropic light-sensitive proteins, channelrodopsins, or metabotropic receptors, rhodopsins, as prosthetic tools.
ANIMAL MODELS AND RECORDING TECHNIQUES
Over the past few years, there have been no apparent changes in the repertoire of model animals used in experiments on designing optogenetic prosthetic technologies. The main animal models are still the mice with an rd (retinal degeneration) phenotype against variable genetic backgrounds, rd1–19 strains, having dysfunctional genes that regulate phototransduction, visual cycle, cellular metabolism, or protein biosynthesis [9]. The most commonly used rd1 strain has a mutation in the Pde6B gene, which leads to dysfunction of phosphodiesterase 6, the key component of the phototransduction cascade. This mouse strain is also genetically heterogeneous, and one of its commercial subpopulations carries an additional mutation in the Gpr179 gene, which encodes a scaffold protein of the transduction cascade signalosome in bipolar cells. This mutation makes its carriers unsuitable for optogenetic bipolar cell prosthetics, thus necessitating an additional genetic modification of animals of this subpopulation in order to return the Gpr179 allele to the wild type [10].
The procedures intended to verify the degree of genetic manipulation success are an important aspect of designing the retinal prosthetic technology. In vivo electroretinogram (ERG) recording from an intact anesthetized animal [11, 12] is still exploited as a method to verify post-prosthetic retinal light sensitivity restoration, which allows functional changes in visual function of a single animal to be tracked over a long period of time. As an ex vivo approach to evaluating retinal light sensitivity, the multichannel recording of ganglion cell activity using a multielectrode array (MEA) takes a dominant position in recent years [13–15]. In this approach, the isolated retina is layered by its ganglion cells over a MEA containing up to 100 electrodes, each of which is able to record the activity of a single or several ganglion cells. In contrast to photoreceptors and bipolar cells, ganglion cells are real neurons that encode a signal entering the brain through optic nerve fibers by changing a spike (action potential) generation frequency. In a healthy retina, a signal is transmitted and processed in the direction from photoreceptors to bipolar and from bipolar to ganglion cells, while the recording of ganglion cell responses allows signals to be analyzed immediately before they enter the brain [16]. Therefore, the application of a MEA is one of the most informative and adequate methods to assess the functional state of the retina and the information the latter is able to communicate to the central nervous system. In this review, when describing the functional consequences of using different prosthetic technologies, we will be referring to the results of MEA-analysis, unless otherwise specified.
VIRUSES OR NANOPARTICLES?
Adeno-associated viruses (AAVs) (see review [17]) are traditional vectors for the effective and safe delivery of a prosthetic genetic material into retinal neurons. This type of carriers has long been widely used for gene therapy of various diseases as a component of antiviral vaccines. The main challenge in using AAVs for retinal optogenetic prosthetics is a small capacity of the viral capsid (up to 4.7 thousand bp) and its low tropism to retinal neurons. Insufficient capsid capacity does not allow its loading with a minimally required set of genetic elements, including a strong promoter of the gene specifically expressed in target retinal cells and the sequence encoding a prosthetic light-sensitive protein. As this drawback is intractable, various compact derivatives of the initial promoter sequence that include a limited number of regulatory sites are usually employed when using AAVs as carriers (for examples, see [18–20]).
The tropism of AAV serotypes (1 to 9) to retinal cells, as well as their ability to traverse the inner limiting (basement) membrane located on the side of ganglion and photoreceptor cells, differ significantly [17]. When injected subretinally (into the space between the retina and the pigment epithelium), AAV1 and 4 serotypes transduce primarily epithelial cells and have a low affinity for retinal neurons [21, 22]. AAV2, 5 and 7 transduce epithelial and photoreceptor cells, while AAV8 and 9 infect the Müller glia as well [21, 22]. However, after intravitreal injection (into the vitreous body), only the AAV2 serotype is able to effectively cross the basement membrane and transduce the inner retinal layers (mainly ganglion cells) [21, 22]. In vitro directed AAV evolution allowed obtaining modified AAV2 serotypes having an increased affinity for retinal neurons and the ability to transduce the cells of intermediate layers, including bipolar cells, more efficiently [23]. For example, multiple substitutions of tyrosine for phenylalanine in AAV2 capsid proteins have been shown to increase transduction efficiency, presumably due to decreasing the proteasomal degradation of viruses by cells [24].
An alternative to viral delivery of a prosthetic material into retinal cells is the use of synthetic nanoparticles of different nature that bind to DNA molecules (for an overview, see [25, 26]). Synthetic vectors have an advantage over their viral counterparts as they have a significantly higher capacity and the ability to transfer plasmids either containing several genes of interest or enriched with regulatory sequences. Although nanoparticles, in contrast to viruses, are unable to implement specific mechanisms of cell penetration and genetic material delivery to the nucleus, researchers have recently learned to modify the particle structure to optimize the mechanisms of their interaction with cells [27–29].
BIPOLAR OR GANGLION CELLS?
As confirmed experimentally, both bipolar and ganglion cells in model animals with photoreceptor degeneration can be successfully used as a target for optogenetic prosthetics (see reviews [6, 30]). In addition, clinical trials have shown that ganglion cells can be employed for prosthetic purposes in humans [31]. The choice of target cells to be artificially imparted with light sensitivity is a paramount step in the overall prosthetics strategy. The main parameters that determine this choice are the availability of a particular cell type for viral transfection, its preservation as degenerative processes in the retina unfold, and the degree of distortion of the natural pathway of visual information dissemination. In a healthy retina, photoreceptors generate in response to light activation a tonic response that hyperpolarizes the cell. This signal, via a glutamate synapse, is summed up with the responses of other photoreceptors and transmitted to an ON- or OFF-bipolar cell, eliciting its tonic depolarization or hyperpolarization, respectively. Eventually, the responses of several bipolar cells are added together and transmitted, both directly and via amacrine cells, to a ganglion cell that responds to the input signal by increasing its spike generation frequency. In the degenerative retina, photoreceptors fall out of the three-link chain of light response generation and signal transmission (photoreceptors → bipolar cells → ganglion cells), thus leaving the possibility of creating both a two-link chain with light-sensitive bipolar cells and a single-link chain with light-sensitive ganglion cells.
Pathological degenerative processes in the retina unfold in several stages (see reviews [32, 33]). At early stages, genetically determined malfunctions in intracellular mechanisms of photoreceptors trigger a cascade of events leading to their death. At this stage, there is also an OFF shift in functional characteristics of bipolar cells that occurs due to changes in the expression profile of glutamate receptors in their dendritic endings [34]. Further pathological processes include the phagocytosis of photoreceptor residues and deafferentation of bipolar cells because of the discontinuation of glutamatergic transmission from photoreceptors and subsequent retraction of their dendrites. Due to these events, functional aberration of bipolar cells progresses, as manifested in an increased expression of ionotropic glutamate receptors in the dendrites of ON-cone bipolar cells, as well as malfunctions in metabotropic receptors [35].
In the transition period, when the rods have already completely degenerated while cones are yet preserved, the dendrites of bipolar cells can proliferate in search of survived photoreceptors to connect to their synaptic terminals. At the last stage, topological remodeling of the retina intensifies, survived cells actively migrate to the locations normally atypical of them, and the basement membrane, largely formed by Müller cells, becomes thicker, thus creating a serious barrier to possible entry of therapeutic or prosthetic agents. Individual bipolar and amacrine cells can migrate into the ganglion cell layer and vice versa, ganglion cells can penetrate into the inner nuclear layer [32]. The observed structural shifts are also accompanied by changes in the transcriptome repertoire of proteins related to cell–cell communication, such as annexin A7 and contactin 1. It has been shown that transcription levels of both proteins in rd1 mice significantly decrease on day 90 of postnatal development [36]. Meanwhile, the transcriptomic profiles, referring to intracellular signaling and basal metabolic processes in bipolar cells, change insignificantly, which allows them to retain the status of promising prosthetic targets.
Another important and negative consequence of retinal functional and topological rearrangement is the emergence of a spontaneous rhythmic activity in most of the survived neurons [37, 38]. This activity creates a noise background and hampers useful signal discrimination, reducing thereby the visual system’s sensitivity. It is commonly believed that the primary cause of this activity is neuronal deafferentation of the inner nuclear layer, while the direct mechanism lies in abnormal membrane potential fluctuations in AII amacrine cells [39, 40]. The latter, cophasally via ON-bipolar cells and antiphasally via the sign-inverting synapse with OFF-bipolar cells, excite periodic activity, respectively, in ON- and OFF-ganglion cells, leading to the emergence of spontaneous electrical activity waves that relatively slowly propagate across the ganglion cell network. This activity manifests itself in the generation of spike bursts with a frequency of about 10 Hz [41]. Pharmacological uncoupling of communication between AII amacrine and ON-bipolar cells through gap junctions leads to a considerable decrease in the spontaneous activity of ganglion cells and an improvement of signal-to-noise ratio [42].
It should be noted that optogenetic prosthetics of bipolar, but not ganglion, cells leaves the signal processing system, which involves amacrine cells, partially operational. Although ganglion cells themselves divide into more than 40 dissimilar functional types that differentially interact and process the incoming information [43, 44], their prosthetics transforms all these functional types into uniform light detectors, thereby significantly disrupting the natural framework of visual information processing before it enters the brain. All the aforesaid indicates that bipolar cell prosthetics recreates the natural framework of visual information processing more adequately compared to ganglion cells prosthetics.
The efficacy of prosthetic transgene delivery to cells of a certain type is largely determined by the choice of the method, intravitreal or subretinal, of how to deliver viral constructs to the retina. It is intravitreal injection that is preferable for transgene delivery to inner retinal neurons, i.e. ganglion and bipolar cells, while subretinal injections are used for the transduction of photoreceptors and pigment epithelium cells (for example, as part of gene therapy, see review [45]). The task of mass ganglion cell transduction is relatively simple because of the maximal proximity of these cells to the vitreous body and, accordingly, the point of vector injection. Bipolar cells occupy the most hard-to-reach position in the retina, which makes them a less attractive target for transgene delivery. However, the advent of modified AAV serotypes with an increased tropism to retinal neurons and, therefore, more efficient penetration into the deep cell layers has allowed this problem to be solved successfully [23, 46].
Attempts to generate selective compact promoters for targeted transgene expression in ON-bipolar cells have been undertaken since 2008 [47] and are usually based on full-length promoters of genes encoding the proteins specific to a certain cell type. Despite the fact that the first developments in this field were unable to provide a sufficient level of selectivity, often admitting the expression of photosensitive proteins in amacrine or ganglion cells [48], at least three constructs providing a highly selective optoprosthetics of ON-bipolar cells on the basis of the Grm6 [49–51] and Pcp2 [49–51] genes can be distinguished to date. A combination of optimized AAV serotypes and selective, powerful and compact promoters allows successful expression to be obtained in 70% of all the retinal bipolar cells [13, 14], with the proportion of transduced cells being significantly higher in rod than in cone bipolar cells. The proportion of non-targeted transgene expression is about 16% and falls on OFF-bipolar cells [14].
Ample data on the safety of the bipolar cell prosthetic technology provide additional evidence for the prospectivity of this approach. It was shown that in rd1 mice, AAV-mediated expression of channelrhodopsin [52, 53] and rhodopsin [53] entailed no apoptosis and inflammation, both local and systemic. On the other hand, no damage or inflammation has also been detected in the macaque retina 3 months after a selective expression of modified channelrhodopsin in ganglion cells [54].
VISUAL OPSINS OR CHANNELRHODOPSINS?
More than ten types of bipolar cells have been identified by now in the mammalian retina, which are subdivided by the type of innervated cells into rod and cone bipolar cells, and by the type of incoming signal processing, into OFF- (sign-preserving) and ON- (sign-inverting) bipolar cells (for review see [55]). A successful prosthetic technology must include an exogenous light-sensitive protein, which allows reproducing the sign of a light-induced change in the bipolar cell membrane potential, as well as the feasibility of selective and efficient expression of this protein in the correct cell type. The technologies developed to date enable ON-bipolar cell prosthetics to be implemented by transfecting these cells with ionotropic or metabotropic light-sensitive proteins. These proteins elicit a light-induced change in the cell membrane potential, reproducing thereby the logic of the normal signaling pathway operation in the retina, as evidenced by the successful experience of visual function prosthetics in model animals [14, 56, 57]. Until now, there have been no attempts of directional OFF-bipolar cell prosthetics, either separately or simultaneously with ON-bipolar cells, although optogenetic tools that make it possible to hyperpolarize cells in response to light stimulation (anion channels) do exist and are successfully used for solving other optogenetic challenges [58]. As for the attempts to use channelrhodopsins and visual opsins for ON-bipolar retinal cell prosthetics in model animals with photoreceptor degeneration, they have long and successfully been made, leading to the emergence of light sensitivity, as supported by the results of various functional tests (for review, see [59]).
When choosing a prosthetic strategy, one of the determinative factors of efficacy is the choice of the type of a prosthetic exogenous light-sensitive protein. Historically, the first successful experiments on optogenetic retinal prosthetics in model animals were implemented using channelrhodopsin [60], and subsequently this protein, along with its modifications, was repeatedly and successfully applied for these purposes [13, 14, 54, 61]. Modified ChrimsonR channelrhodopsin with its red-shifted absorption maximum was successfully used for retinal ganglion cells prosthetics [31]. The great advantage of using channelrhodopsin is a relative autonomy of this approach, as the prosthetic effect only relies on the expression of a single exogenous protein. An alternative to channelrhodopsin as a retinal prosthetic tool is light-sensitive metabotropic receptors, such as optic opsins or melanopsin. In this case, it is assumed that the exogenous receptor is incorporated into the prosthetized cell’s own signaling system. The postsynaptic ON-bipolar cell signaling cascade, triggered by the mGluR6 metabotropic glutamatergic receptor and regulating the conductance of TRPM1 channels, is most commonly considered as a suitable system for receptor incorporation (see review [62]). The metabotropic light-sensitive receptor can be used in an unmodified form (rod rhodopsin [63, 64]; human cone opsin [46]; melanopsin [65, 66]), or its molecule can be modified to better adapt to the endogenous signaling cascade. In the latter case, a chimeric receptor protein can be used, in which the native intracellular domain, responsible for the interaction with signaling G proteins, is substituted for the mGluR6 receptor domain [56, 67].
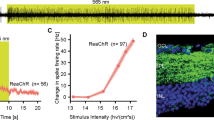
When prosthetizing bipolar cells with one or another type of proteins, the outcome may differ significantly by the final light sensitivity of the retina. When the viral vector carrying similar genetic constructs is injected at equal concentrations, the sensitivity of ON-bipolar cells prosthetized with channelrhodopsin was 2.5 orders of magnitude lower than in prosthetizing with the metabotropic receptor (rat rhodopsin) [63]. A similar ratio of sensitivities also follows from other works, in which bipolar cells were prosthetized with channelrhodopsins [13, 61] and demonstrated threshold sensitivities in the range of 1013–1015 photons/cm2/s, or with metabotropic opsins [42, 51, 64] and showed threshold sensitivities of 1011–1012 photons/cm2/s (see the summary diagram in Fig. 1). The explanation of such a difference lies most likely in the level of bipolar cell depolarization in terms of a single light quantum absorbed. Absorption of a single quantum by the channelrhodopsin molecule leads to the opening of a single channel, channelrhodopsin itself, whereas single-quantum activation of the metabotropic receptor molecule triggers an intracellular transduction cascade, leading to the opening of numerous membrane cation channels. Accordingly, in the second case, there is a greater shift in the membrane potential, which means a greater light sensitivity of a prosthetized cell.
Bipolar cell prosthetics with metabotropic opsins leads to the emergence of light sensitivity which covers up to 5 orders of intensity, whereas the use of channelrhodopsins allows creating a dynamic range of only 2 orders of intensity [42, 63]. It occurs, specifically, due to a higher slope of the stimulus intensity–light response curve in the case of channelrhodopsin prosthetics than with the use of the metabotropic receptor [51]. At the same time, bipolar cell prosthetics with metabotropic opsins creates at the level of individual ganglion cells a dynamic range of sensitivity of no more than 4 orders of light stimulation intensity. The additional order of light sensitivity adjustment is achieved due to superimposition and incomplete overlap of sensitivities of many different ganglion cells [42].
The shape of the prosthetized retina’s response to a stimulation by a prolonged step of light is expectedly different from that of the healthy retina. When prosthetizing ON-bipolar cells with rhodopsin, the response at the level of ganglion cells shows a slower response kinetics compared to the healthy retina (longer time to peak and slower off response). At the same time, the response demonstrates the signs of light adaptation in the artificial phototransduction cascade, manifested in response acceleration as the stimulus intensity increases, which is also characteristic of a healthy retina [63]. It should also be noted that some ganglion cells (about 7% of their total number) responded to stimulation by not an increase but a decrease in spike activity, which indicates a partial restoration of OFF-pathways in visual signal processing.
A summary diagram of threshold sensitivity values in an optogenetically prosthetized degenerative retina after optogenetic prosthetization according to data from various sources. The data (minimum light stimulus intensity that elicits a retinal response) are clustered depending on which type of cells underwent prosthetics (bipolar or ganglionic) and which type of a prosthetic light-sensitive protein (channelrhodopsin or visual opsin) were used. References: retinal ganglion cells, channelrhodopsins (RGC, ChR) [14, 54, 68–70]; ganglion cells, visual opsins (RGC, Vis.Ops) [66, 71]; bipolar cells, channelrhodopsins (BC, ChR) [13, 14, 52, 63, 72, 73]; bipolar cells, visual opsins (BC, Vis.Ops) [51, 56, 63, 64]. Abbreviations: ChR2—channelrhodopsin-2; CatCh/CoChR/ReaCHR—modified channelrhodopsins; Rho—rod rhodopsin; RGC—retinal ganglion cells; MW-Opn—green-sensitive cone opsin; Mln—melanopsin.
CONCLUSIONS
In this review, we have attempted to describe relatively recent achievements in developing optogenetic technologies to prosthetize the retina that has undergone neurodegeneration. New evidence has been obtained that the use of adeno-associated viruses as transduction vectors, as well as the induction of rhodopsin or channelrhodopsin expression in retinal cells, is safe both for the retina [52, 53, 74] and for the organism as a whole [52]. Moreover, in animal models, the induction of rhodopsin and channelrhodopsin expression is stable over many months [13, 52, 74], which allows us to hope for a stable effect of prosthetic protein expression in humans in case this method is introduced into clinical practice.
To date, an effective toolkit for the delivery of genetic material to survived retinal cells has been created and tested in animals with photoreceptor degeneration. A combination of certain AAV serotypes with the latest compact promoters makes it possible to achieve high selectivity and efficiency of transduction of the retinal cell type chosen for prosthetics in model animals (rodents [50, 51], dogs [74, 75], primates [54, 76], reviewed in [77]). A direct transfer of such an AAV serotype–promoter combination to human retinal prosthetic technology seems to be impossible, and, therefore, a highly specific and efficient delivery of genetic material into human cells will require the development and subsequent validation of appropriate species-specific promoters (see [18]).
Currently, an unambiguous choice in favor of bipolar or ganglion cell prosthetics, as well as the choice of channelrhodopsins or rhodopsins as a prosthetic material, does not seem obvious. Bipolar cell prosthetics recreates a more natural signaling pathway in the retina, and it seems likely that the visual system will adapt more easily to this prosthetic option. On the other hand, the proximal location of ganglion cells makes them more accessible to viral vectors, despite all the recent achievements in creating serotypes that penetrate deep into the retina. Direct measurements confirm that channelrhodopsin prosthetization of ganglion cells allows obtaining a significantly higher sensitivity than with channelrhodopsin prosthetization of bipolar cells [14].
In all works on bipolar cell prosthetics with channelrhodopsin, the threshold of retinal light sensitivity is no less than 1013 photons/cm2/s, including using modern modified forms of this protein having an increased conductance and delayed channel shutdown [14, 78]. A low sensitivity necessitates the constant use of high lighting levels, which can lead to light-induced retinal damage. The use of metabotropic opsins in all cases allows creating light sensitivity approximately two orders of magnitude higher than with the use of channelrhodopsins and thus reducing the light load on the retina [51, 63]. In addition, it has been shown that in ON-bipolar cells, the rhodopsin-based artificial transduction cascade is able to adapt to lighting levels. Both of these circumstances suggest that future promising prosthetic tools must be developed on the basis of metabotropic light-sensitive receptors.
REFERENCES
Mills JO, Jalil A, Stanga PE (2017) Electronic retinal implants and artificial vision: journey and present. Eye (Lond) 31(10): 1383–1398. https://doi.org/10.1038/eye.2017.65
Bloch E, Luo Y, da Cruz L (2019) Advances in retinal prosthesis systems. Therapeutic advances in ophthalmology 11: 2515841418817501. https://doi.org/10.1177/2515841418817501
Ahuja AK, Behrend MR (2013) The Argus™ II retinal prosthesis: factors affecting patient selection for implantation. Progress in retinal and eye research 36: 1–23. https://doi.org/10.1016/j.preteyeres.2013.01.002
Firsov M (2019) Perspectives for the Optogenetic Prosthetization of the Retina. Neuroscience and Behavioral Physiology 49: 192–198. https://doi.org/10.1007/s11055-019-00714-2
Kirpichnikov MP, Ostrovskiy MA (2015) Optogenetics and prosthetic treatment of retinal degeneration. Vestn Oftalmol 131(3): 99–111. https://doi.org/10.17116/oftalma2015131399-111
Ostrovsky MA, Kirpichnikov MP (2019) Prospects of Optogenetic Prosthesis of the Degenerative Retina of the Eye. Biochemistry (Mosc) 84(5): 479–490. https://doi.org/10.1134/S0006297919050031
Scholl HP, Strauss RW, Singh MS, Dalkara D, Roska B, Picaud S, Sahel J-A (2016) Emerging therapies for inherited retinal degeneration. Sci Transl Med 8(368): 368rv6. https://doi.org/10.1126/scitranslmed.aaf2838
Chaffiol A, Duebel J (2018) Mini-Review: Cell Type-Specific Optogenetic Vision Restoration Approaches. Advances in experimental medicine and biology 1074: 69–73. https://doi.org/10.1007/978-3-319-75402-4_9
Collin GB, Gogna N, Chang B, Damkham N, Pinkney J, Hyde LF, Stone L, Naggert JK, Nishina PM, Krebs MP (2020) Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss. Cells 9(4): 931. https://doi.org/10.3390/cells9040931
Nishiguchi KM, Carvalho LS, Rizzi M, Powell K, Holthaus SM, Azam SA, Duran Y, Ribeiro J, Luhmann UFO, Bainbridge JWB, Smith AJ, Ali RR (2015) Gene therapy restores vision in rd1 mice after removal of a confounding mutation in Gpr179. Nat Commun 6: 6006. https://doi.org/10.1038/ncomms7006
A L, Zou T, He J, Chen X, Sun D, Fan X, Xu H (2019) Rescue of Retinal Degeneration in rd1 Mice by Intravitreally Injected Metformin. Frontiers in molecular neuroscience 12: 102. https://doi.org/10.3389/fnmol.2019.00102
Dalke C, Löster J, Fuchs H, Gailus-Durner V, Soewarto D, Favor J, Neuhäuser-Klaus A, Pretsch W, Gekeler F, Shinoda K, Zrenner E, Meitinger T, Hrabé de Angelis M, Graw J (2004) Electroretinography as a screening method for mutations causing retinal dysfunction in mice. Investigative ophthalmology and visual science 45(2): 601–609. https://doi.org/10.1167/iovs.03-0561
Mace E, Caplette R, Marre O, Sengupta A, Chaffiol A, Barbe P, Desrosiers M, Bamberg E, Sahel J-A, Picaud S, Duebel J (2015) Targeting channelrhodopsin-2 to ON-bipolar cells with vitreally administered AAV Restores ON and OFF visual responses in blind mice. Mol Ther 23(1): 7–16. https://doi.org/10.1038/mt.2014.154
Lu Q, Ganjawala TH, Krstevski A, Abrams GW, Pan ZH (2020) Comparison of AAV-Mediated Optogenetic Vision Restoration between Retinal Ganglion Cell Expression and ON Bipolar Cell Targeting. Molecular therapy Methods and clinical development 18: 15–23. https://doi.org/10.1016/j.omtm.2020.05.009
Tu HY, Matsuyama T (2020) Multielectrode Array Recording of Mouse Retinas Transplanted with Stem Cell-Derived Retinal Sheets. Methods Mol Biol 2092: 207–220. https://doi.org/10.1007/978-1-0716-0175-4_15
Reinhard K, Tikidji-Hamburyan A, Seitter H, Idrees S, Mutter M, Benkner B, Münch TA (2014) Step-by-step instructions for retina recordings with perforated multi electrode arrays. PloS one 9(8): e106148. https://doi.org/10.1371/journal.pone.0106148
Planul A, Dalkara D (2017) Vectors and Gene Delivery to the Retina. Annu Rev Vis Sci 3: 121–140. https://doi.org/10.1146/annurev-vision-102016-061413
Juttner J, Szabo A, Gross-Scherf B, Morikawa RK, Rompani SB, Hantz P, Szikra T, Esposti F, Cowan CS, Bharioke A, Patino-Alvarez CP, Keles Ö, Kusnyerik A, Azoulay Th, Hartl D, Krebs AR, Schübeler D, Hajdu RI, Lukats A, Nemeth J, Nagy ZZ, Wu K-Ch, Wu R-H, Xiang L, Fang X-L, Jin Z-B, Goldblum D, Hasler PW, Scholl HPN, Krol J, Roska B (2019) Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat Neurosci 22(8): 1345–1356. https://doi.org/10.1038/s41593-019-0431-2
de Leeuw CN, Dyka FM, Boye SL, Laprise S, Zhou M, Chou AY, Lisa Borretta, McInerny SC, Banks KG, Elodie Portales-Casamar, Swanson MI, D’Souza CA, Boye ShE, Jones SJM, Holt RA, Goldowitz D, Hauswirth WW, Wasserman WW, Simpson EM (2014) Targeted CNS Delivery Using Human MiniPromoters and Demonstrated Compatibility with Adeno-Associated Viral Vectors. Molecular Therapy Methods and Clinical Development 1: 5. https://doi.org/10.1038/mtm.2013.5
Korecki AJ, Cueva-Vargas JL, Fornes O, Agostinone J, Farkas RA, Hickmott JW, Lam SL, Mathelier A, Zhou M, Wasserman WW, Di Polo A, Simpson EM (2021) Human MiniPromoters for ocular-rAAV expression in ON bipolar, cone, corneal, endothelial, Muller glial, and PAX6 cells. Gene Ther 28(6): 351–372. https://doi.org/10.1038/s41434-021-00227-z
Auricchio A, Kobinger G, Anand V, Hildinger M, O’Connor E, Maguire AM, Wilson JM, Bennett J (2001) Exchange of surface proteins impacts on viral vector cellular specificity and transduction characteristics: the retina as a model. Hum Mol Genet 10(26): 3075–3081. https://doi.org/10.1093/hmg/10.26.3075
Allocca M, Mussolino C, Garcia-Hoyos M, Sanges D, Iodice C, Petrillo M, Vandenberghe LH, Wilson JM, Marigo V, Surace EM, Auricchio A (2007) Novel adeno-associated virus serotypes efficiently transduce murine photoreceptors. J Virol 81(20): 11372–11380. https://doi.org/10.1128/JVI.01327-07
Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV (2013) In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med 5(189): 189ra76. https://doi.org/10.1126/scitranslmed.3005708
Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, Chiodo V, Neeley AW, Govindasamy L, Bennett A, Agbandje-McKenna M, Zhong L, Li B, Jayandharan GR, Srivastava A, Lewin AS, Hauswirth WW (2011) Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther 19(2): 293–301. https://doi.org/10.1038/mt.2010.234
Adijanto J, Naash MI (2015) Nanoparticle-based technologies for retinal gene therapy. Eur J Pharm Biopharm 95(Pt B): 353–367. https://doi.org/10.1016/j.ejpb.2014.12.028
Rotov AY, Romanov IS, Tarakanchikova YV, Astakhova LA (2021) Application Prospects for Synthetic Nanoparticles in Optogenetic Retinal Prosthetics. Journal of Evolutionary Biochemistry and Physiology 57(6): 1333–1350. https://doi.org/10.1134/S0022093021060132
Cardoso MM, Peça IN, Roque AC (2012) Antibody-conjugated nanoparticles for therapeutic applications. Current medicinal chemistry 19(19): 3103–3127. https://doi.org/10.2174/092986712800784667
Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF (2013) Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Advanced drug delivery reviews 65(1): 121–138. https://doi.org/10.1016/j.addr.2012.09.041
Batabyal S, Kim S, Wright W, Mohanty S (2021) Layer-specific nanophotonic delivery of therapeutic opsin-encoding genes into retina. Exp Eye Res 205: 108444. https://doi.org/10.1016/j.exer.2021.108444
Baker CK, Flannery JG (2018) Innovative Optogenetic Strategies for Vision Restoration. Front Cell Neurosci 12: 316. https://doi.org/10.3389/fncel.2018.00316
Sahel JA, Boulanger-Scemama E, Pagot C, Arleo A, Galluppi F, Martel JN (2021) Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 27(7): 1223-1229. https://doi.org/10.1038/s41591-021-01351-4. Epub 2021 May 24.
Jones BW, Kondo M, Terasaki H, Lin Y, McCall M, Marc RE (2012) Retinal remodeling. Jpn J Ophthalmol 56(4): 289–306. https://doi.org/10.1007/s10384-012-0147-2. Epub 2012 May 30.
Jones BW, Pfeiffer RL, Ferrell WD, Watt CB, Marmor M, Marc RE (2016) Retinal remodeling in human retinitis pigmentosa. Exp Eye Res 150: 149–165. https://doi.org/10.1016/j.exer.2016.03.018
Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, et al. (2007) Neural reprogramming in retinal degeneration. Investigative ophthalmology and visual science 48(7): 3364–3371. https://doi.org/10.1167/iovs.07-0032
Chua J, Fletcher EL, Kalloniatis M (2009) Functional remodeling of glutamate receptors by inner retinal neurons occurs from an early stage of retinal degeneration. J Comp Neurol 514(5): 473–491. https://doi.org/10.1002/cne.22029
Gilhooley MJ, Hickey DG, Lindner M, Palumaa T, Hughes S, Peirson SN (2021) ON-bipolar cell gene expression during retinal degeneration: Implications for optogenetic visual restoration. Exp Eye Res 207: 108553. https://doi.org/10.1016/j.exer.2021.108553. Epub 2021 Mar 31.
Margolis DJ, Detwiler PB (2011) Cellular origin of spontaneous ganglion cell spike activity in animal models of retinitis pigmentosa. J Ophthalmol 2011: 507037. Epub 2010 Sep 29. https://doi.org/10.1155/2011/507037
Trenholm S, Awatramani GB (2015) Origins of spontaneous activity in the degenerating retina. Front Cell Neurosci 9: 277. https://doi.org/10.3389/fncel.2015.00277. eCollection 2015.
Borowska J, Trenholm S, Awatramani GB (2011) An intrinsic neural oscillator in the degenerating mouse retina. J Neurosci 31(13): 5000–5012. https://doi.org/10.1523/JNEUROSCI.5800-10.2011
Margolis DJ, Gartland AJ, Singer JH, Detwiler PB (2014) Network oscillations drive correlated spiking of ON and OFF ganglion cells in the rd1 mouse model of retinal degeneration. PloS one 9(1): e86253. https://doi.org/10.1371/journal.pone.0086253
Stasheff SF (2008) Emergence of sustained spontaneous hyperactivity and temporary preservation of OFF responses in ganglion cells of the retinal degeneration (rd1) mouse. J Neurophysiol 99(3): 1408–1421. https://doi.org/10.1152/jn.00144.2007
Eleftheriou CG, Cehajic-Kapetanovic J, Martial FP, Milosavljevic N, Bedford RA, Lucas RJ (2017) Meclofenamic acid improves the signal to noise ratio for visual responses produced by ectopic expression of human rod opsin. Mol Vis 23: 334–345.
Kolb H, Linberg KA, Fisher SK (1992) Neurons of the human retina: a Golgi study. J Comp Neurol 318(2): 147–187. https://doi.org/10.1002/cne.903180204
Kim US, Mahroo OA, Mollon JD, Yu-Wai-Man P (2021) Retinal Ganglion Cells-Diversity of Cell Types and Clinical Relevance. Frontiers in neurology 12: 661938. https://doi.org/10.3389/fneur.2021.661938
Rotov AY, Nikolaeva DA, Astakhova LA, Firsov ML (2020) Virus Vectors for Optogenetic Prosthetization of the Retina. Neuroscience and Behavioral Physiology 50(3): 358–366. https://doi.org/10.1007/s11055-020-00911-4
McClements ME, Staurenghi F, Visel M, Flannery JG, MacLaren RE, Cehajic-Kapetanovic J (2021) AAV Induced Expression of Human Rod and Cone Opsin in Bipolar Cells of a Mouse Model of Retinal Degeneration. BioMed research international 2021: 1–8. https://doi.org/10.1155/2021/4014797
Kim DS, Matsuda T, Cepko CL (2008) A core paired-type and POU homeodomain-containing transcription factor program drives retinal bipolar cell gene expression. J Neurosci 28(31): 7748–7764. https://doi.org/10.1523/JNEUROSCI.0397-08.2008
van Wyk M, Hulliger EC, Girod L, Ebneter A, Kleinlogel S (2017) Present Molecular Limitations of ON-Bipolar Cell Targeted Gene Therapy. Front Neurosci 11: 161. https://doi.org/10.3389/fnins.2017.00161
Lu Q, Ganjawala TH, Ivanova E, Cheng JG, Troilo D, Pan ZH (2016) AAV-mediated transduction and targeting of retinal bipolar cells with improved mGluR6 promoters in rodents and primates. Gene Ther 23(8-9): 680-689. https://doi.org/10.1038/gt.2016.42
Hulliger EC, Hostettler SM, Kleinlogel S (2020) Empowering Retinal Gene Therapy with a Specific Promoter for Human Rod and Cone ON-Bipolar Cells. Mol Ther Methods Clin Dev 17: 505–519. eCollection 2020 Jun 12. https://doi.org/10.1016/j.omtm.2020.03.003
Gilhooley MJ, Lindner M, Palumaa T, Hughes S, Peirson SN, Hankins MW (2022) A systematic comparison of optogenetic approaches to visual restoration. Mol Ther Methods Clin Dev 25: 111–123. eCollection 2022 Jun 9. https://doi.org/10.1016/j.omtm.2022.03.003
Doroudchi MM, Greenberg KP, Liu J, Silka KA, Boyden ES, Lockridge JA (2011) Virally delivered channelrhodopsin-2 safely and effectively restores visual function in multiple mouse models of blindness. Mol Ther 19(7): 1220–1229. https://doi.org/10.1038/mt.2011.69. Epub 2011 Apr 19.
Wright P, Rodgers J, Wynne J, Bishop PN, Lucas RJ, Milosavljevic N (2021) Viral Transduction of Human Rod Opsin or Channelrhodopsin Variants to Mouse ON Bipolar Cells Does Not Impact Retinal Anatomy or Cause Measurable Death in the Targeted Cells. Int J Mol Sci 22(23): 13111. https://doi.org/10.3390/ijms222313111
Chaffiol A, Caplette R, Jaillard C, Brazhnikova E, Desrosiers M, Dubus E, et al. (2017) A New Promoter Allows Optogenetic Vision Restoration with Enhanced Sensitivity in Macaque Retina. Mol Ther 25(11): 2546–2560. https://doi.org/10.1016/j.ymthe.2017.07.011
Euler T, Haverkamp S, Schubert T, Baden T (2014) Retinal bipolar cells: elementary building blocks of vision. Nat Rev Neurosci 15(8): 507–519. https://doi.org/10.1038/nrn3783.
van Wyk M, Pielecka-Fortuna J, Lowel S, Kleinlogel S (2015) Restoring the ON Switch in Blind Retinas: Opto-mGluR6, a Next-Generation, Cell-Tailored Optogenetic Tool. PLoS Biol 13(5): e1002143. https://doi.org/10.1371/journal.pbio.1002143
Schilardi G, Kleinlogel S (2021) Two Functional Classes of Rod Bipolar Cells in the Healthy and Degenerated Optogenetically Treated Murine Retina. Front Cell Neurosci 15: 809531. eCollection 2021. https://doi.org/10.3389/fncel.2021.809531
Khabou H, Garita-Hernandez M, Chaffiol A, Reichman S, Jaillard C, Brazhnikova E, et al. (2018) Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 3(2): 96029. https://doi.org/10.1172/jci.insight.96029
Simunovic MP, Shen W, Lin JY, Protti DA, Lisowski L, Gillies MC (2019) Optogenetic approaches to vision restoration. Exp Eye Res 178: 15–26. https://doi.org/10.1016/j.exer.2018.09.003
Bi A, Cui J, Ma YP, Olshevskaya E, Pu M, Dizhoor AM (2006) Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50(1): 23–33. https://doi.org/10.1016/j.neuron.2006.02.026.
Lindner M, Gilhooley MJ, Peirson SN, Hughes S, Hankins MW (2021) The functional characteristics of optogenetic gene therapy for vision restoration. Cell Mol Life Sci 78(4): 1597–1613. Epub 2020 Jul 29. https://doi.org/10.1007/s00018-020-03597-6
Martemyanov KA, Sampath AP (2017) The Transduction Cascade in Retinal ON-Bipolar Cells: Signal Processing and Disease. Annual review of vision science 3: 25–51. https://doi.org/10.1146/annurev-vision-102016-061338
Gaub BM, Berry MH, Holt AE, Isacoff EY, Flannery JG (2015) Optogenetic Vision Restoration Using Rhodopsin for Enhanced Sensitivity. Mol Ther 23(10): 1562–1571. https://doi.org/10.1038/mt.2015.121
Cehajic-Kapetanovic J, Eleftheriou C, Allen AE, Milosavljevic N, Pienaar A, Bedford R (2015) Restoration of Vision with Ectopic Expression of Human Rod Opsin. Curr Biol 25(16): 2111–2122. Epub 2015 Jul 30. https://doi.org/10.1016/j.cub.2015.07.029
Lin B, Koizumi A, Tanaka N, Panda S, Masland RH (2008) Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proceedings of the National Academy of Sciences of the United States of America 105(41): 16009–16014. https://doi.org/10.1073/pnas.0806114105
De Silva SR, Barnard AR, Hughes S, Tam SKE, Martin C, Singh MS (2017) Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proceedings of the National Academy of Sciences of the United States of America 114(42): 11211–11226. https://doi.org/10.1073/pnas.1701589114
Ballister ER, Rodgers J, Martial F, Lucas RJ (2018) A live cell assay of GPCR coupling allows identification of optogenetic tools for controlling Go and Gi signaling. BMC biology 16(1): 10. https://doi.org/10.1186/s12915-017-0475-2
Thyagarajan S, van Wyk M, Lehmann K, Löwel S, Feng G, Wässle H (2010) Visual function in mice with photoreceptor degeneration and transgenic expression of channelrhodopsin 2 in ganglion cells. J Neurosci 30(26): 8745–8758. https://doi.org/10.1523/jneurosci.4417-09.2010
Tomita H, Sugano E, Isago H, Hiroi T, Wang Z, Ohta E (2010) Channelrhodopsin-2 gene transduced into retinal ganglion cells restores functional vision in genetically blind rats. Exp Eye Res 90(3): 429–436. https://doi.org/10.1016/j.exer.2009.12.006
Sengupta A, Chaffiol A, Mace E, Caplette R, Desrosiers M, Lampic M (2016) Red-shifted channelrhodopsin stimulation restores light responses in blind mice, macaque retina, and human retina. EMBO Mol Med 8(11): 1248–1264. https://doi.org/10.15252/emmm.201505699
Berry MH, Holt A, Salari A, Veit J, Visel M, Levitz J, Aghi K, Gaub BM,Sivyer B, Flannery JG, Isacoff EY (2019) Restoration of high-sensitivity and adapting vision with a cone opsin. Nat Commun 10(1): 1221. https://doi.org/10.1038/s41467-019-09124-x
Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B (2008) Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nature neuroscience 11(6): 667–675. https://doi.org/10.1038/nn.2117
Cronin T, Vandenberghe LH, Hantz P, Juttner J, Reimann A, Kacso AE, Huckfeldt RM, Busskamp V, Kohler H, Lagali PS, Roska B, Bennett J (2014) Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol Med 6(9): 1175–1190. https://doi.org/10.15252/emmm.201404077
Ameline B, Tshilenge KT, Weber M, Biget M, Libeau L, Caplette R, Mendes-Madeira A, Provost N, Guihal C, Picaud S, Moullier P, Pichard V, Cronin T, Isiegas C (2017) Long-term expression of melanopsin and channelrhodopsin causes no gross alterations in the dystrophic dog retina. Gene Ther 24(11): 735–741. https://doi.org/10.1038/gt.2017.63
Beltran WA (2009) The use of canine models of inherited retinal degeneration to test novel therapeutic approaches. Veterinary ophthalmology 12(3): 192–204. https://doi.org/10.1111/j.1463-5224.2009.00694.x
Chaffiol A, Provansal M, Joffrois C, Blaize K, Labernede G, Goulet R, Burban E, Brazhnikova E, Duebel J, Pouget P, Sahel JA, Picaud S, Arcizet F, Gauvain G (2022) In vivo optogenetic stimulation of the primate retina activates the visual cortex after long-term transduction. Molecular therapy Methods & clinical development 24: 1–10. https://doi.org/10.1016/j.omtm.2021.11.009
Winkler PA, Occelli LM, Petersen-Jones SM (2020) Large Animal Models of Inherited Retinal Degenerations: A Review. Cells 9(4): 882. https://doi.org/10.3390/cells9040882
Ganjawala TH, Lu Q, Fenner MD, Abrams GW, Pan ZH (2019) Improved CoChR Variants Restore Visual Acuity and Contrast Sensitivity in a Mouse Model of Blindness under Ambient Light Conditions. Mol Ther 27(6): 1195–1205. Epub 2019 Apr 9. https://doi.org/10.1016/j.ymthe.2019.04.002
Funding
This work was supported by funds from the Ministry of Science and Higher Education of the Russian Federation, agreement no. 075-15-2022-296, to support the World-Class Research Center “Pavlovian Center”.
Author information
Authors and Affiliations
Contributions
Both authors equally contributed to literature data collection and analysis, as well as the writing of this review article.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 6, pp. 457–467https://doi.org/10.31857/S0044452922060092.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rotov, A.Y., Firsov, M.L. Optogenetic Prosthetization of Retinal Bipolar Cells. J Evol Biochem Phys 58, 1675–1686 (2022). https://doi.org/10.1134/S0022093022060011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060011