Abstract
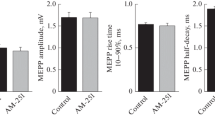
The effects of VU 0238429, an allosteric modulator of muscarinic acetylcholine (ACh) M5 receptor, on the amplitude and temporal parameters of spontaneous and evoked endplate potentials, as well as on the force of muscle contractions in different modes of motor nerve stimulation, were studied. It was shown that M5 cholinoreceptors regulate the quantal release of acetylcholine from motor nerve endings in the mouse diaphragm. Specifically, M5 activation increases the level of ACh release during low-frequency nerve stimulation (0.5 Hz) and enhances synaptic depression at 70 Hz. Pharmacological potentiation of M5 cholinoreceptors exerts a depressing effect on the force of skeletal muscle contractions at stimulation frequencies in the range from 0.5 to 70 Hz, which must be considered when developing new drugs aimed at affecting M5 cholinoreceptors.


Similar content being viewed by others
REFERENCES
Wess J, Eglen RM, Gautam D (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6:721–733. https://doi.org/10.1038/nrd2379
Yamada M, Basile AS, Fedorova I, Zhang W, Duttaroy A, Cui Y, Lamping KG, Faraci FM, Deng CX, Wess J (2003) Novel insights into M5 muscarinic acetylcholine receptor function by the use of gene targeting technology. Life Sci 74:345–353. https://doi.org/10.1016/j.lfs.2003.09.022
Langmead CJ, Watson J, Reavill C (2008) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 117:232–243. https://doi.org/10.1016/j.pharmthera.2007.09.009
Araya R, Noguchi T, Yuhki M, Kitamura N, Higuchi M, Saido TC, Seki K, Itohara S, Kawano M, Tanemura K, Takashima A, Yamada K, Kondoh Y, Kanno I, Wess J, Yamada M (2006) Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol Dis 24:334–344. https://doi.org/10.1016/j.nbd.2006.07.010
Wright MC, Potluri S, Wang X, Dentcheva E, Gautam D, Tessler A, Wess J, Rich MM, Son YJ (2009) Distinct muscarinic acetylcholine receptor subtypes contribute to stability and growth, but not compensatory plasticity, of neuromuscular synapses. J Neurosci 29:14942–14955. https://doi.org/10.1523/jneurosci.2276-09.2009
Tsentsevitsky AN, Kovyazina IV, Nurullin LF, Nikolsky EE (2017) Muscarinic cholinoreceptors (M1-, M2-, M3- and M4-type) modulate the acetylcholine secretion in the frog neuromuscular junction. Neurosci Lett 649:62–69. https://doi.org/10.1016/j.neulet.2017.04.015
Bridges TM, Marlo JE, Niswender CM, Jones CK, Jadhav SB, Gentry PR, Plumley HC, Weaver CD, Conn PJ, Lindsley CW (2009) Discovery of the first highly M5-preferring muscarinic acetylcholine receptor ligand, an M5 positive allosteric modulator derived from a series of 5-trifluoromethoxy N-benzyl isatins. J Med Chem 52:3445-3448. https://doi.org/10.1021/jm900286j
Ruiz R, Cano R, Casañas JJ, Gaffield MA, Betz WJ, Tabares L (2011) Active zones and the readily releasable pool of synaptic vesicles at the neuromuscular junction of the mouse. J Neurosci 31:2000-2008. https://doi.org/10.1523/JNEUROSCI.4663-10.2011
Nathanson NM (2008) Synthesis, trafficking, and localization of muscarinic acetylcholine receptors. Pharmacol Ther 119: 33–43. https://doi.org/10.1016/j.pharmthera.2008.04.006
Malomouzh AI, Mukhtarov MR, Nikolsky EE, Vyskočil F (2007) Muscarinic M1 acetylcholine receptors regulate the non-quantal release of acetylcholine in the rat neuromuscular junction via NO-dependent mechanism. J Neurochem 102:2110–2117. https://doi.org/10.1111/j.1471-4159.2007.04696.x
Tsentsevitsky AN, Zakyrjanova GF, Petrov AM, Kovyazina IV (2020) Breakdown of phospholipids and the elevated nitric oxide are involved in M3 muscarinic regulation of acetylcholine secretion in the frog motor synapse. Biochem Biophys Res Commun 524: 589–594. https://doi.org/10.1016/j.bbrc.2020.01.112
Garcia N, Santafé MM, Salon I, Lanuza MA, Tomàs J (2005) Expression of muscarinic acetylcholine receptors (M1-, M2-, M3- and M4-type) in the neuromuscular junction of the newborn and adult rat. Histol Histopathol 20:733–743. https://doi.org/10.14670/HH-20.733
Oliveira L, Timóteo MA, Correia-de-Sá P (2009) Negative crosstalk between M1 and M2 muscarinic autoreceptors involves endogenous adenosine activating A1 receptors at the rat motor endplate. Neurosci Lett 459:127–131. https://doi.org/10.1016/j.neulet.2009.05.001
Levey AI (1993) Immunological localization of m1-m5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci 52:441–448. https://doi.org/10.1016/0024-3205(93)90300-r
Bukharaeva EA, Kim KK, Nikol’skii EE, Vyskochil F (2000) Synchronization of evoked secretion of quanta of mediator as a mechanism facilitating the action of sympathomimetics. Neurosci Behav Physiol 30:139–146. https://doi.org/10.1007/BF02463151
Malomouzh AI, Arkhipova SS, Nikolsky EE, Vyskočil F (2011) Immunocytochemical demonstration of M(1) muscarinic acetylcholine receptors at the presynaptic and postsynaptic membranes of rat diaphragm endplates. Physiol Res 60:185–188. https://doi.org/10.33549/physiolres.932131
Newman Z, Malik P, Wu TY, Ochoa C, Watsa N, Lindgren C (2007) Endocannabinoids mediate muscarine-induced synaptic depression at the vertebrate neuromuscular junction. Eur J Neurosci 25:1619–1630. https://doi.org/10.1111/j.1460-9568.2007.05422.x
Felder CC, Ma AL, Briley EM, Axelrod J (1993) Muscarinic acetylcholine receptor subtypes associated with release of Alzheimer amyloid precursor derivatives activate multiple signal transduction pathways. Ann NY Acad Sci 695:15–18. https://doi.org/10.1111/j.1749-6632.1993.tb23020.x
Guo J, Schofield GG (2003) Activation of muscarinic m5 receptors inhibits recombinant KCNQ2/KCNQ3 K+ channels expressed in HEK293T cells. Eur J Pharmacol 462:25–32. https://doi.org/10.1016/s0014-2999(03)01323-2
Cumbay MG, Watts VJ (2005) Galphaq potentiation of adenylate cyclase type 9 activity through a Ca2+/calmodulin-dependent pathway. Biochem Pharmacol 69:1247–1256. https://doi.org/10.1016/j.bcp.2005.02.001
Cantrell AR, Ma JY, Scheuer T, Catterall WA (1996) Muscarinic modulation of sodium current by activation of protein kinase C in rat hippocampal neurons. Neuron 16:1019–1026. https://doi.org/10.1016/s0896-6273(00)80125-7
Pemberton KE, Jones SV (1997) Inhibition of the L-type calcium channel by the five muscarinic receptors (m1-m5) expressed in NIH 3T3 cells. Pflugers Arch 433:505-514. https://doi.org/10.1007/s004240050306
Mitchell JF, Silver A (1963) The spontaneous release of acetylcholine from the denervated hemidiaphragm of the rat. J Physiol 165:117–129. https://doi.org/10.1113/jphysiol.1963.sp007046
Krnjevic K, Straughan DW (1964) The release of acetylcholine from the denervated rat diaphragm. J Physiol 170:371–378. https://doi.org/10.1113/jphysiol.1964.sp007337
Molenaar PC, Polak RL (1980) Acetylcholine synthesizing enzymes in frog skeletal muscle. J Neurochem 35:1021–1025. https://doi.org/10.1111/j.1471-4159.1980.tb07855.x
Tucek S (1982) The synthesis of acetylcholine in skeletal muscles of the rat. J Physiol 322:53–69. https://doi.org/10.1113/jphysiol.1982.sp014022
Krivoi II (2002) Mechanisms of the non-neurotransmitter actions of acetylcholine in the neuromuscular apparatus. Neurosci Behav Physiol 32:149–156. https://doi.org/10.1023/a:1013975324963
Vyskočil F, Malomouzh AI, Nikolsky EE (2009) Non-quantal acetylcholine release at the neuromuscular junction. Physiol Res 58:763–784. https://doi.org/10.33549/physiolres.931865
Funding
Electrophysiological studies were supported by the Russian Foundation for Basic Research grant No. 20-04-00571; the experiments on recording muscle contractions were implemented within a governmental assignment to the Kazan Institute of Biochemistry and Biophysics, FRC Kazan Scientific Center of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
The basic idea and experimental design (I.V.K., A.I.M.); data collection (A.A.Kh., N.S.F.); data processing (I.V.K., A.A.Kh., N.S.F., A.I.M.); writing and editing a manuscript (I.V.K., A.I.M.).
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 1, pp. 98–108https://doi.org/10.31857/S0869813922010083.
Rights and permissions
About this article
Cite this article
Kovyazina, I.V., Khamidullina, A.A., Fedorov, N.S. et al. Effects of VU 0238429, an Allosteric Modulator of M5 Cholinoreceptors, on Neuromuscular Transmission in the Mouse Diaphragm. J Evol Biochem Phys 58, 149–157 (2022). https://doi.org/10.1134/S0022093022010136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022010136