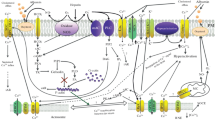
Abstract
The cAMP-dependent signaling cascades play the key role in regulation of fertility of spermatozoa. Synthesis of cAMP in spermatozoa is realized both by soluble, and by transmembrane (membrane-bound) forms of adenylyl cyclases (AC). For the recent years numerous data appeared about the presence in spermatozoa at different stages of their maturation of a wide spectrum isoforms of membrane-bound AC and their regulation by hormones and hormone-like substances via the coupled to B-proteins receptors (GPCR). Agonists of GPCR in spermatozoa can be adenosine, biogenic amines, peptide hormones, odorants. Study of structural-functional organization and regulatory properties of AC of the signal system in spermatozoa is of great practical significance for reproductive technologies, as via the membrane-bound AC forms and signal cascades there are controlled such processes as motility and chemotaxis of spermatozoa, their capability for capacitation acrosomal reaction. In the review there are summarized and analyzed data on functioning and role of AC of signal system in spermatozoa of human and vertebrate animals and are discussed achievements and unsolved problems in this field.
Similar content being viewed by others
Abbreviations
- AdenR:
-
adenosine receptor
- ACE:
-
angiotensin-converting enzyme
- AP:
-
adrenergic receptor
- AC:
-
adenylyl cyclase
- ACSS:
-
adenylyl cyclase signaling system
- GIDP:
-
5′-guanosine imidodiphosphate
- GTPγS:
-
guanosine-5′-O-thiotriphosphate
- PT:
-
pertussis toxin
- MR:
-
melatonin receptor
- sAC:
-
soluble adenylyl cyclase
- SR:
-
serotonin receptor
- tmAC:
-
transmembrane (membrane-bound) adenylyl cyclase
- CT:
-
cholera toxin
- FPP:
-
fertilization promoting peptide
- GPCR:
-
G protein-coupled receptor)
- MRP4:
-
multidrug resistance associated protein 4
References
Abou-haila, A. and Tulsiani, D.R., Signal transduction pathways that regulate sperm capacitation and the acrosome reaction, Arch. Biochem. Biophys., 2009, vol. 485, pp. 72–81.
Bailey, J.L., Factors regulating sperm capacitation, Syst. Biol. Reprod. Med., 2010, vol. 56, pp. 334–348.
Signorelli, J., Diaz, E.S., and Morales, P., Kinases, phosphatases and proteases during sperm capacitation, Cell. Tissue Res., 2012, vol. 349, pp. 765–782.
Kamenetsky, M., Middelhaufe, S., Bank, E.M., Levin, L.R., Buck, J., and Steegborn, C., Molecular details of cAMP generation in mammalian cells: a tale of two systems, J. Mol. Biol., 2006, vol. 362, pp. 623–639.
Sinha, S.C. and Sprang, S.R., Structures, mechanism, regulation and evolution of class III nucleotidyl cyclases, Rev. Physiol. Biochem. Pharmacol., 2006, vol. 157, pp. 105–140.
Shpakov, A.O., Structure-functional organization of adenylyl cyclases of unicellular eukaryotes and molecular mechanisms of their regulation, Cell Tissue Biol., 2007, vol. 1, pp. 97–114.
Shpakov, A.O. and Pertseva, M.N., Signaling systems of lower eukaryotes and their evolution, Int. Rev. Cell Mol. Biol., 2008, vol. 269, pp. 151–282.
Buck, J., Sinclair, M.L., Schapal, L., Cann, M.J., and Levin, L.R., Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals, Proc. Natl. Acad. Sci. USA, 1999, vol. 96, pp. 79–84.
Chen, Y., Cann, M.J., Litvin, T.N., Iourgenko, V., Sincler, M.L., Levin, L.R., and Buck, J., Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor, Science, 2000, vol. 289, pp. 625–628.
Acin-Perez, R., Salazar, E., Brosel, S., Yang, H., Schon, E.A., and Manfredi, G., Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects, EMBO Mol. Med., 2009, vol. 1, pp. 392–406.
Kumar, S., Kostin, S., Flacke, J.P., Reusch, H.P., and Ladilov, Y., Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells, J. Biol. Chem., 2009, vol. 284, pp. 14 760–14 768.
Chen, J., Levin, L.R., and Buck, J., Role of soluble adenylyl cyclase in the heart, Am. J. Physiol., 2012, vol. 302, pp. H538–543.
Jaiswal, B.S. and Conti, M., Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase, J. Biol. Chem., 2001, vol. 276, pp. 31 698–31 708.
Jaiswal, B.S. and Conti, M., Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa, Proc. Natl. Acad. Sci. USA, 2003, vol. 100, pp. 10 676–10 681.
Derkach, K.V., Shpakov, A.O., and Gryaznov, A.Yu., Reguratory properties of cytosol and membrane-bound adenylyl cyclases in fractions of spermatozoa with different motility, Dokl. RAN, 2012, vol. 445, no. 4, pp. 468–470.
Shpakov, A.O., Derkach, K.V., Gryaznov, A.Yu. and Motovilova, N.O., Regulatory properties of adenylyl cyclase and guanylyl cyclasein human spermatozoa, Zh. Evol. Biokhim. Fiziol., 2013, vol. 49, no. 1, pp. 30–38.
Geng, W., Wang, Z., Zhang, J., Reed, B.Y., Pak, C.Y., and Moe, O.W., Cloning and characterization of the human soluble adenylyl cyclase, Am. J. Physiol., 2005, vol. 288, pp. C1305–1316.
Tresguerres, M., Levin, L.R., and Buck, J., Intracellular cAMP signaling by soluble adenylyl cyclase, Kidney Int., 2011, vol. 79, pp. 1277–1288.
Shpakov, A.O. and Derkach, K.V., Soluble forms of adenylyl cyclases of spermatozoa, Tsitologiya, 2014, vol. 56, no. 1, pp. 5–13.
Bentley, J.K., Garbers, D.L., Domino, S.E., Noland, T.D., and Van Dop, C., Spermatozoa contain a guanine nucleotide-binding protein ADP-ribosylated by pertussis toxin, Biochem. Biophys. Res. Commun., 1986, vol. 138, pp. 728–734.
Kopf, G.S., Woolkalis, M.J., and Gerton, G.L., Evidence for a guanine nucleotide-binding regulatory protein in invertebrate and mammalian sperm: identification by islet-activating protein-catalyzed ADP-ribosylation and immunochemical methods, J. Biol. Chem., 1986, vol. 261, pp. 7327–7331.
Monks, N.J., Stein, D.M., and Fraser, L.R., Adenylate cyclase activity of mouse sperm during capacitation in vitro: effect of calcium and a GTP analogue, Int. J. Androl., 1986, vol. 9, pp. 67–76.
Endo, Y., Lee, M.A., and Kopf, G.S., Evidence for the role of a guanine nucleotide-binding regulatory protein in the zona pellucida-induced mouse sperm acrosome reaction, Dev. Biol., 1987, vol. 119, pp. 210–216.
Shpakov, A.O., Molecular determinants in receptors of serpentine type responsible for their coupling to heterotrimeric G-proteins, Tsitologiya, 2002, vol. 44, no. 3, pp. 242–258.
Shpakov, A.O., Participation of charged amino acid residues of cytoplasmic loops of receptors in process of transduction of hormonal signal, Zh. Evol. Biokhim. Fiziol., 2003, vol. 39, no. 3, pp. 205–217.
Gudermann, T., Kalkbrenner, F., and Schultz, G., Diversity and selectivity of receptor-G protein interaction, Annu. Rev. Pharmacol. Toxicol., 1996, vol. 36, pp. 429–460.
Ostrom, R.S., Bogard, A.S., Gros, R., and Feldman, R.D., Choreographing the adenylyl cyclase signalosome: sorting out the partners and the steps, Naunyn Schmiedebergs Arch. Pharmacol., 2012, vol. 385, pp. 5–12.
Seamon, K.B. and Daly, J.W., Forskolin: a unique diterpene activator of cyclic AMP-generating systems, J. Cyclic Nucleotide Res., 1981, vol. 7, pp. 201–224.
Vijayaraghavan, S. and Hoskins, D.D., Forskolin stimulates bovine epididymal sperm motility and cyclic AMP levels, J. Cyclic Nucleotide Protein Phosphor. Res., 1985, vol. 10, pp. 499–510.
Fraser, L.R., Pondel, M.D., and Vinson, G.P., Calcitonin, angiotensin II and FPP significantly modulate mouse sperm function, Mol. Hum. Reprod., 2001, vol. 7, pp. 245–253.
Baxendale, R.W. and Fraser, L.R., Evidence for multiple distinctly localized adenylyl cyclase isoforms in mammalian spermatozoa, Mol. Reprod. Dev., 2003, vol. 66, pp. 181–189.
Spehr, M., Schwane, K., Riffell, J.A., Barbour, J., Zimmer, R.K., Neuhaus, E.M., and Hatt, H., Particulate adenylate cyclase plays a key role in human sperm olfactory receptor-mediated chemotaxis, J. Biol. Chem., 2004, vol. 279, pp. 40194–40203.
Wertheimer, E., Krapf, D., Vega-Beltran, J.L., Sánchez-Cárdenas, C., Navarrete, F., Haddad, D., Escoffier, J., Salicioni, A.M., Levin, L.R., Buck, J., Mager, J., Darszon, A., and Visconti, P.E., Compartmentalization of distinct cAMP signaling pathways in mammalian sperm, J. Biol. Chem., 2013, PMID: 24129574. doi: 10.1074/jbc. M113.489476.
Derkach, K.V., Shpakov, A.O., and Gryaznov, A.Yu., Functional activity of adenylyl cyclase and guanylyl cyclase in kuman spermatozoa with different motility, Tsitologiya, 2013, vol. 55, no. 2, pp. 123–130.
Leclerc, P. and Kopf, G.S., Evidence for the role of heterotrimeric guanine nucleotide-binding regulatory proteins in the regulation of the mouse sperm adenylyl cyclase by the egg’s zona pellucida, J. Androl., 1999, vol. 20, pp. 126–134.
Leclerc, P. and Kopf, G.S., Mouse sperm adenylyl cyclase: general properties and regulation by the zona pellucida, Biol. Reprod., 1995, vol. 52, pp. 1227–1233.
Wade, M.A., Roman, S.D., Jones, R.C., and Aitken, R.J., Adenylyl cyclase isoforms in rat testis and spermatozoa from the cauda epididymidis, Cell. Tissue Res., 2003, vol. 314, pp. 411–419.
Wang, H. and Storm, D.R., Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system, Mol. Pharmacol., 2003, vol. 63, pp. 463–468.
Wong, S.T., Trinh, K., Hacker, B., Chan, G.C., Lowe, G., Gaggar, A., Xia, Z., Gold, G.H., and Storm, D.R., Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice, Neuron, 2000, vol. 27, pp. 487–497.
Livera, G., Xie, F., Garcia, M.A., Jaiswal, B., Chen, J., Law, E., Storm, D.R., and Conti, M., Inactivation of the mouse adenylyl cyclase 3 gene disrupts male fertility and spermatozoon function, Mol. Endocrinol., 2005, vol. 19, pp. 1277–1290.
Defer, N., Marinx, O., Poyard, M., Lienard, M.O., Jegou, B., and Hanoune, J., The olfactory adenylyl cyclase type 3 is expressed in male germ cells, FEBS Lett., 1998, vol. 424, pp. 216–220.
Gautier-Courteille, C., Salanova, M., and Conti, M., The olfactory adenylyl cyclase III is expressed in rat germ cells during spermiogenesis, Endocrinology, 1998, vol. 139, pp. 2588–2599.
Spehr, M., Gisselmann, G., Poplawski, A., Riffell, J.A., Wetzel, C.H., Zimmer, R.K., and Hatt, H., Identification of a testicular odorant receptor mediating human sperm chemotaxis, Science, 2003, vol. 299, pp. 2054–2058.
Olsson, P. and Laska, M., Human male superiority in olfactory sensitivity to the sperm attractant odorant bourgeonal, Chem. Senses, 2010, vol. 35, pp. 427–432.
Kjeldmand, L., Salazar, L.T., and Laska, M., Olfactory sensitivity for sperm-attractant aromatic aldehydes: a comparative study in human subjects and spider monkeys, J. Comp. Physiol., 2011, vol. 197, pp. 15–23.
Glassner, M., Jones, J., Kligman, I., Woolkalis, M.J., Gerton, G.L., and Kopf, G.S., Immunocytochemical and biochemical characterization of guanine nucleotide-binding regulatory proteins in mammalian spermatozoa, Dev. Biol., 1991, vol. 146, pp. 438–450.
Karnik, N.S., Newman, S., Kopf, G.S., and Gerton, G.L., Developmental expression of G protein alpha subunits in mouse spermatogenic cells: evidence that Gαi is associated with the developing acrosome, Dev. Biol., 1992, vol. 152, pp. 393–402.
Ward, C.R., Storey, B.T., and Kopf, G.S., Selective activation of Gi1 and Gi2 in mouse sperm by the zona pellucida, the egg’s extracellular matrix, J. Biol. Chem., 1994, vol. 269, pp. 13254–13258.
Ning, X., Ward, C.R., and Kopf, G.S., Activation of a Gi protein in digitonin/cholate-solubilized membrane preparations of mouse sperm by the zona pellucida, an egg-specific extracellular matrix, Mol. Reprod. Dev., 1995, vol. 40, pp. 355–363.
Fraser, L.R. and Adeoya-Osiguwa, S.A., Modulation of adenylyl cyclase by FPP and adenosine involves stimulatory and inhibitory adenosine receptors and G-proteins, Mol. Reprod. Dev., 1999, vol. 53, pp. 459–471.
Fraser, L.R., Adeoya-Osiguwa, S.A., and Baxendale, R.W., First messenger regulation of capacitation via G protein-coupled mechanisms: a tale of serendipity and discovery, Mol. Hum. Reprod., 2003, vol. 9, pp. 739–748.
Minelli, A., Allegrucci, C., Piomboni, P., Mannucci, R., Lluis, C., and Franco, R., Immunolocalization of A1 adenosine receptors in mammalian spermatozoa, J. Histochem. Cytochem., 2000, vol. 48, pp. 1163–1171.
Adeoya-Osiguwa, S.A., and Fraser, L.R., Capacitation state-dependent changes in adenosine receptors and their regulation of adenylyl cyclase/cAMP, Mol. Reprod. Dev., 2002, vol. 63, pp. 245–255.
Osycka-Salut, C., Diez, F., Burdet, J., Gervasi, M.G., Franchi, A., Bianciotti, L.G., Davio, C., and Perez-Martinez, S., Cyclic AMP efflux, via MRPs and A1 adenosine receptors, is critical for bovine sperm capacitation, Mol. Hum. Reprod., 2013, PMID: 23907162. doi: 10.1093/molehr/gat053.
Minelli, A., Bellezza, I., Collodel, G., and Fredholm, B.B., Promiscuous coupling and involvement of protein kinase C and extracellular signal-regulated kinase 1/2 in the adenosine A1 receptor signalling in mammalian spermatozoa, Biochem. Pharmacol., 2008, vol. 75, pp. 931–941.
Minelli, A., Liguori, L., Bellazza, I., Mannucci, R., Johansson, B., and Fredholm, B.B., Involvement of A1 adenosine receptors in the acquisition of fertilizing capacity, J. Androl., 2004, vol. 25, pp. 286–292.
Fredholm, B.B., Chen, J.F., Masino, S.A., and Vaugeois, J.M., Action of adenosine at its receptors in the CNS: insights from knockouts and drugs, Annu. Rev. Pharmacol. Toxicol., 2005, vol. 45, pp. 385–412.
Burnett, L.A., Blais, E.M., Unadkat, J.D., Hille, B., Tilley, S.L., and Babcock, D.F., Testicular expression of Adora3i2 in Adora3 knockout mice reveals a role of mouse A3Ri2 and human A3Ri3 adenosine receptors in sperm, J. Biol. Chem., 2010, vol. 285, pp. 33662–33670.
Vijayaraghavan, S. and Hoskins, D.D., Regulation of bovine sperm motility and cyclic adenosine 3′,5′-monophosphate by adenosine and its analogues, Biol. Reprod., 1986, vol. 34, pp. 468–477.
Aitken, R.J., Mattei, A., and Irvine, S., Paradoxical stimulation of human sperm motility by 2-deoxyadenosine, J. Reprod. Fertil., 1986, vol. 78, pp. 515–527.
Fenichel, P., Gharib, A., Emiliozzi, C., Donzeau, M., and Menezo, Y., Stimulation of human sperm during capacitation in vitro by an adenosine agonist with specificity for A2 receptors, Biol. Reprod., 1996, vol. 54, pp. 1405–1411.
Allegrucci, C., Liguori, L., and Minelli, A., Stimulation by N6-cyclopentyladenosine of A1 adenosine receptors, coupled to Gαi2 protein subunit, has a capacitative effect on human spermatozoa, Biol. Reprod., 2001, vol. 64, pp. 1653–1659.
Adeoya-Osiguwa, S.A. and Fraser, L.R., Fertilization promoting peptide and adenosine, acting as first messengers, regulate cAMP production and consequent protein tyrosine phosphorylation in a capacitation-dependent manner, Mol. Reprod. Dev., 2000, vol. 57, pp. 384–392.
Somanath, P.R., Jack, S.L., and Vijayaraghavan, S., Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation, J. Androl., 2004, vol. 25, pp. 605–617.
Mededovic, S. and Fraser, L.R., Angiotensin II stimulates cAMP production and protein tyrosine phosphorylation in mouse spermatozoa, Reproduction, 2004, vol. 127, pp. 601–612.
Fraser, L.R., Fertilization promoting peptide: an important regulator of sperm function in vivo?, Rev. Reprod., 1998, vol. 3, pp. 151–154.
Adeoya-Osiguwa, S.A. and Fraser, L.R., Calcitonin acts as a first messenger to regulate adenylyl cyclase/cAMP and mammalian sperm function, Mol. Reprod. Dev., 2003, vol. 65, pp. 228–236.
Fraser, L.R., Adeoya-Osiguwa, S., Baxendale, R.W., Mededovic, S., and Osiguwa, O.O., First messenger regulation of mammalian sperm function via adenylyl cyclase/cAMP, J. Reprod. Dev., 2005, vol. 51, pp. 37–46.
Yanagimachi, R., Mammalian fertilization, In: The Physiology of Reproduction, 2nd Edition, Knobil, E. and Neil, J.D., Eds., N.Y.: Raven Press, 1994, pp. 189–317.
Wennemuth, G., Babcock, D.F., and Hille, B., Distribution and function of angiotensin II receptors in mouse spermatozoa, Andrologia, 1999, vol. 31, pp. 323–325.
Sabeur, K., Vo, A.T., and Ball, B.O., Effects of angiotensin II on the acrosome reaction in equine spermatozoa, J. Reprod. Fertil., 2000, vol. 120, pp. 135–142.
Ball, B.A., Gravance, C.G., Wessel, M.T., and Sabeur, K., Activity of angiotensin-converting enzyme (ACE) in reproductive tissues of the stallion and effects of angiotensin II on sperm motility, Theriogenology, 2003, vol. 59, pp. 901–914.
Köhn, F.M., Dammshäuser, I., Neukamm, C., Renneberg, H., Siems, W.E., Schill, W.B., and Aumüller, G., Ultrastructural localization of angiotensinconverting enzyme in ejaculated human spermatozoa, Hum. Reprod., 1998, vol. 13, pp. 604–610.
Shibahara, H., Kamata, M., Hu, J., Nakagawa, H., Obara, H., Kondoh, N., Shima, H., and Sato, I., Activity of testis angiotensin converting enzyme (ACE) in ejaculated human spermatozoa, Int. J. Androl., 2001, vol. 24, pp. 295–299.
Costa, D.S. and Thundathil, J.C., Characterization and activity of angiotensin-converting enzyme in Holstein semen, Anim. Reprod. Sci., 2012, vol. 133, pp. 35–42.
Xu, J., Baulding, J., and Palli, S.R., Proteomics of Tribolium castaneum seminal fluid proteins: identification of an angiotensin-converting enzyme as a key player in regulation of reproduction, J. Proteomics., 2013, vol. 78, pp. 83–93.
Fraser, L.R., The role of small molecules in sperm capacitation, Theriogenology, 2008, vol. 70, pp. 1356–1359.
Cornett, L.E. and Meizel, S., Stimulation of in vitro activation and the acrosome reaction of hamster spermatozoa by catecholamines, Proc. Natl. Acad. Sci. USA, 1978, vol. 75, pp. 4954–4958.
Way, A.L. and Killian, G.J., Capacitation and induction of the acrosome reaction in bull spermatozoa with norepinephrine, J. Androl., 2002, vol. 23, pp. 352–357.
Way, A.L. and Killian, G.J., Sperm binding, in vitro fertilization, and in vitro embryonic development of bovine oocytes fertilized with spermatozoa incubated with norepinephrine, Anim. Reprod. Sci., 2006, vol. 96, pp. 1–9.
Fujinoki, M., Melatonin-enhanced hyperactivation of hamster sperm, Reproduction, 2008, vol. 136, pp. 533–541.
Fujinoki, M., Serotonin-enhanced hyperactivation of hamster sperm, Reproduction, 2011, vol. 142, pp. 255–266.
Shpakov, A.O., Structural-functional characteristics of neuronal serotonin receptors and mo;ecular mechanisms of their functional coupling to G-proteins, Neirokhimiya, 2009, vol. 26, no. 1, pp. 5–18.
Jiménez-Trejo, F., Tapia-Rodríguez, M., Cerbón, M., Kuhn, D.M., Manjarrez-Gutiérrez, G., Mendoza-Rodríguez, C.A., and Picazo, O., Evidence of 5-HT components in human sperm: implications for protein tyrosine phosphorylation and the physiology of motility, Reproduction, 2012, vol. 144, pp. 677–685.
Jiménez-Trejo, F., León-Galván, M.Á., Martínez-Méndez, L.A., Tapia-Rodríguez, M., Mendoza-Rodríguez, C.A., González-Santoyo, I., López-Wilchis, R., Vela-Hinojosa, C., Baranda-Avila, N., and Cerbón, M., Serotonin in testes of bat Myotis velifer during annual reproductive cycle: expression, localization, and content variations, J. Exp. Zool. A. Ecol. Genet. Physiol., 2013, vol. 319, pp. 249–258.
Takuwa, Y., Okamoto, Y., Yoshioka, K., and Takuwa, N., Sphingosine-1-phosphate signaling in physiology and diseases, Biofactors, 2012, vol. 38, pp. 329–337.
Suhaiman, L., De Blas, G.A., Obeid, L.M., Darszon, A., Mayorga, L.S., and Belmonte, S.A., Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis, J. Biol. Chem., 2010, vol. 285, pp. 16302–16314.
Belmonte, S.A. and Suhaiman, L., Optimized protocols to analyze sphingosine-1-phosphate signal transduction pathways during acrosomal exocytosis in human sperm, Methods Mol. Biol., 2012, vol. 874, pp. 99–128.
Zhang, B.L., Li, Y., Ding, J.H., Dong, F.L., Hou, Y.J., Jiang, B.C., Shi, F.X., and Xu, Y.X., Sphingosine 1-phosphate acts as an activator for the porcine Gpr3 of constitutively active G proteincoupled receptors, J. Zhejiang Univ. Sci. B., 2012, vol. 13, pp. 555–566.
Fukuda, N., Yomogida, K., Okabe, M., and Touhara, K., Functional characterization of a mouse testicular olfactory receptor and its role in chemosensing and in regulation of sperm motility, J. Cell Sci., 2004, vol. 117, pp. 5835–5845.
Vanderhaeghen, P., Schurmans, S., Vassart, G., and Parmentier, M., Olfactory receptors are displayed on dog mature sperm cells, J. Cell. Biol., 1993, vol. 123, pp. 1441–1452.
Vanderhaeghen, P., Schurmans, S., Vassart, G., and Parmentier, M., Specific repertoire of olfactory receptor genes in the male germ cells of several mammalian species, Genomics, 1997, vol. 39, pp. 239–246.
Walensky, L.D., Roskams, A.J., Lefkowitz, R.J., Snyder, S.H., and Ronnett, G.V., Odorant receptors and desensitization proteins colocalize in mammalian sperm, Mol. Med., 1995, vol. 1, pp. 130–141.
Spehr, M. and Hatt, H., A potential role of odorant receptor agonists and antagonists in the treatment of infertility and contraception, Curr. Opin. Investig. Drugs, 2005, vol. 6, pp. 364–368.
Meyer, D., Voigt, A., Widmayer, P., Borth, H., Huebner, S., Breit, A., Marschall, S., de Angelis, M.H., Boehm, U., Meyerhof, W., Gudermann, T., and Boekhoff, I., Expression of Tas1 taste receptors in mammalian spermatozoa: functional role of Tas1r1 in regulating basal Ca2+ and cAMP concentrations in spermatozoa, PLoS One, 2012, vol. 7. e32354.
Behrens, M., Meyerhof, W., Hellfritsch, C., and Hofmann, T., Sweet and umami taste: natural products, their chemosensory targets, and beyond, Angew. Chem. Int. Ed. Engl., 2011, vol. 50, pp. 2220–2242.
Fehr, J., Meyer, D., Widmayer, P., Borth, H.C., Ackermann, F., Wilhelm, B., Gudermann, T., and Boekhoff, I., Expression of the G-protein α-subunit gustducin in mammalian spermatozoa, J. Comp. Physiol., 2007, vol. 193, pp. 21–34.
Clapp, T.R., Trubey, K.R., Vandenbeuch, A., Stone, L.M., Margolskee, R.F., Chaudhari, N., and Kinnamon, S.C., Tonic activity of Gα-gustducin regulates taste cell responsivity, FEBS Lett., 2008, vol. 582, pp. 3783–3787.
Etkovitz, N., Tirosh, Y., Chazan, R., Jaldety, Y., Daniel, L., Rubinstein, S., and Breitbart, H., Bovine sperm acrosome reaction induced by G protein-coupled receptor agonists is mediated by epidermal growth factor receptor transactivation, Dev. Biol., 2009, vol. 334, pp. 447–457.
Breitbart, H. and Etkovitz, N., Role and regulation of EGFR in actin remodeling in sperm capacitation and the acrosome reaction, Asian J. Androl., 2011, vol. 13, pp. 106–110.
Revelli, A., Ghigo, D., Moffa, F., Massobrio, M., and Tur-Kaspa, I., Guanylate cyclase activity and sperm function, Endocr. Rev., 2002, vol. 23, pp. 484–494.
Naz, R.K. and Sellamuthu, R., Receptors in spermatozoa: are they real?, J. Androl., 2006, vol. 27, pp. 627–636.
Xie, F., Garcia, M.A., Carlson, A.E., Schuh, S.M., Babcock, D.F., Jaiswal, B.S., Gossen, J.A., Esposito, G., van Duin, M., and Conti, M., Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization, Dev. Biol., 2006, vol. 296, pp. 353–362.
Anderson, R.A., Feathergill, K.A., Chany, C.J., Jain, S., and Krunic, A., Nitric oxide-dependent human acrosomal loss induced by PPCM (SAMMA) and by nitric oxide donors occurs by independent pathways: basis for synthesis of an improved contraceptive microbicide, J. Androl., 2009, vol. 30, pp. 168–182.
Miraglia, E., De Angelis, F., Gazzano, E., Hassanpour, H., Bertagna, A., Aldieri, E., Revelli, A., and Ghigo, D., Nitric oxide stimulates human sperm motility via activation of the cyclic GMP/protein kinase G signaling pathway, Reproduction, 2011, vol. 141, pp. 47–54.
Shpakov, A.O., Derkach, K.V., and Pertseva, M.N., Hormonal system of lower eukaryots, Tsitologiya, 2003, vol. 45, no. 3, pp. 223–234.
Shpakov, A.O., Structural-functional organization of adenylyl cyclases of unicellular eukaryots, Structural-organization of adenylyl cyclases of unicellular eukaryots, Tsitologiya, 2007, vol. 49, no. 2, pp. 91–106.
Shpakov, A.O., Receptors of serpentine type and heterotrimeric G-proteins of yeast fungi: structural-functional organization and molecular mechanisms of action, Heterotrimeric proteins of yeast fungi, Zh. Evol. Biokhim. Fiziol., 2007, vol. 43, no. 1, pp. 3–23.
Shpakov, A.O., Signal molecules of bacteria of nonpeptide nature of QS-type, Microbiol., 2009, vol. 78, no. 2, pp. 163–175.
Shpakov, A.O., Peptide autoinductors of bacteria, Microbiol., 2009, vol. 78, no. 3, pp. 291–303.
Pertseva, M.N. and Shpakov, A.O., The prokaryotic origin and evolution of eukaryotic chemosignaling systems, Neurosci. Behav. Physiol., 2009, vol. 39, pp. 793–804.
Shpakov, A.O. and Pertseva, M.N., Systems of signaling transduction of eukaryots, Zh. Evol. Biokhim. Fiziol., 2008, vol. 44, no. 2, pp. 113–130.
Oberholzer, M., Bregy, P., Marti, G., Minca, M., Peier, M., and Seebeck, T., Trypanosomes and mammalian sperm: one of a kind?, Trends Parasitol., 2007, vol. 23, pp. 71–77.
Derkach, K.V., Shpakov, A.O., Kuznetsova, L.A., Plesneva, S.A., Uspenskaya, Z.I., and Pertseva, M.N., Hormonesensensitive adenylyl cyclase system of infusorium Dileptus anser, Tsitologiya, 2002, vol. 44, no. 11, pp. 1129–1134.
Derkach, K.V., Shpakov, A.O., Kuznetsova, L.A., Irlina, I.S., Plesneva, S.A., and Pertseva, M.M., Regulation of adenylyl cyclase system of infusorium Tetrahymena pyriformis by hormonal and nonhormonal agents and nonhormonal agents and its dependence on level of basal activity of adenylyl cyclase, Zh. Evol. Biokhim. Fiziol., 2003, vol. 39, no. 4, pp. 332–338.
Shpakov, A.O., Derkach, K.V., Uspenskaya, Z.I., Shpakova, E.A., Kuznetsova, L.A., Plesneva, S.A., and Pertseva, M.N., Molecular mechanisms of regulator action of adreneractivity of adenylyl cyclase signaling system of infusoria Dileptus anser and Tetrahymena pyriformis, Tsitologiya, 2004, vol. 46, no. 4, pp. 317–325.
Shpakov, A.O., Derkach, K.V., Uspenskaya, Z.I., and Pertseva, M.N., Stimulation of adenylyl cyclase by cyclic adenosine monophosphate in cellular culture of infusorian Dileptus anser, Dokl. Akad. Nauk, 2009, vol. 424, no. 2, pp. 270–272.
Derkach, K.V., Shpakov, A.O., Uspenskaya, Z.I., and Yudin, A.L., Functional characteristics of calcium-sensitive adenylyl cyclase of infusorian Tetrahymena pyriformis, Tsitologiya, 2010, vol. 52, no. 11, pp. 967–976.
Shpakov, A.O., Derkach, K.V., and Uspenskaya, Z.I., Effect of natural amino acid and sugars on activity of cyclases of infusoria Tetrahymena pyriformis and Dileptus anser, Zh. Evol. Biokhim. Fiziol., 2011, vol. 47, no. 2, pp. 128–135.
Zhu, L. and Inaba, K., Lipid rafts function in Ca2+ signaling responsible for activation of sperm motility and chemotaxis in the ascidian Ciona intestinalis, Mol. Reprod. Dev., 2011, vol. 78, pp. 920–929.
Bookbinder, L.H., Moy, G.W., and Vacquier, V.D., Identification of sea urchin sperm adenylate cyclase, J. Cell. Biol., 1990, vol. 111, pp. 1859–1866.
Beltrán, C., Vacquier, V.D., Moy, G., Chen, Y., Buck, J., Levin, L.R., and Darszon, A., Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction, Biochem. Biophys. Res. Commun., 2007, vol. 358, pp. 1128–1135.
Tubbs, C. and Thomas, P., Progestin signaling through an olfactory G protein and membrane progestin receptor-α in Atlantic croaker sperm: potential role in induction of sperm hypermotility, Endocrinology, 2009, vol. 150, pp. 473–484.
O’Brien, E.D., Krapf, D., Cabada, M.O., Visconti, P.E., and Arranz, S.E., Transmembrane adenylyl cyclase regulates amphibian sperm motility through protein kinase A activation, Dev. Biol., 2011, vol. 350, pp. 80–88.
Baldi, E., Krausz, C., and Forti, G., Nongenomic actions of progesterone on human spermatozoa, Trends Endocrinol. Metab., 1995, vol. 6, pp. 198–205.
Teves, M.E., Guidobaldi, H.A., Uñates, D.R., Sanchez, R., Miska, W., Publicover, S.J., Morales Garcia, A.A., and Giojalas, L.C., Molecular mechanism for human sperm chemotaxis mediated by progesterone, PLoS One, 2009, vol. 4, pp. e8211.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.O. Shpakov, K.V. Derkach, 2014, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2014, Vol. 50, No. 4, pp. 255–268.
Rights and permissions
About this article
Cite this article
Shpakov, A.O., Derkach, K.V. Functional role of membrane-bound adenylyl cyclases and coupled to them receptors and G-proteins in regulation of fertility of spermatozoa. J Evol Biochem Phys 50, 286–302 (2014). https://doi.org/10.1134/S0022093014040024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093014040024