The exciton absorption spectrum of microscopic CuCl crystals grown in a transparent dielectric matrix has been studied. The size of the microscopic crystals was varied in a controlled manner from several tens of angstroms to hundreds of angstroms. There is a short-wave shift (of up to 0.1 eV) of the exciton absorption lines, caused by a quantum size effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Size effects in semiconductors have recently attracted considerable interest. Most of the experiments which have been reported have used quasi-two-dimensional structures grown by molecular epitaxy [1], MOS structures [2], etc. In this letter we report the discovery and a spectroscopic study of a new class of objects that exhibit size effects: three-dimensional microscopic crystals of semiconducting compounds grown in a transparent dielectric matrix.
For the experiments we used multicomponent silicate glasses, with an initial composition including compounds of copper and chlorine at a concentration of the order of 1%. It was found recently [3] that when such glasses are heated to a high temperature the characteristic exciton-absorption spectra of CuCl crystals appear in the transparency region of the matrix. These spectra directly imply that a microscopically disperse crystalline phase of CuCl forms in the glass as a result of the phase decomposition of a supersaturated solid solution which occurs in the matrix during the heat treatment.
The diffusive phase decomposition of the supersaturated solid solution in the recondensation stage (the stage in which the supersaturation is slight, and the large nucleating regions grow at the expense of the dissolution of small regions) has been studied in detail in a theoretical paper by Lifshitz and Slezov [4]. They showed that the average radius of the new-phase nuclei, \(\bar {a}\), increases with the time t in accordance with the following asymptotic law during the recondensation growth:
where D is the diffusion coefficient, and α is a coefficient which is determined by the interfacial surface tension. The concentration of the new phase in the matrix remains constant during the growth. Lifshitz and Slezov also showed that during the recondensation growth of the new phase a steady-state size distribution of the particles is formed. This distribution does not depend on the initial conditions, and its dispersion is relatively small. An analytic expression was derived for this distribution.
Figure 1 shows experimental values of the average radius of the microscopic CuCl crystals precipitated in the matrix by the heat treatment, plotted as a function of the heating time for two different temperatures. The sizes of the microscopic crystals were determined by the method of small-angle X-ray scattering [5] in the approximation of monodisperse spherical particles. We see from this figure that the experimental data do in fact reveal the behavior \(\bar {a}\) ~ t1/3, which indicates a recondensation nature of the growth of the microscopic crystals of the semiconducting phase in the matrix. The slope of the lines in Fig. 1, i.e., the growth rate of the crystals, depends strongly on the heat-treatment temperature and is determined by the diffusion coefficient D, which is an exponential function of the temperature. Accordingly, by choosing the heat-treatment conditions (the temperature and time) appropriately during the growth of semiconducting crystals in a glassy matrix, it is possible to synthesize microscopic crystals of any prespecified size over a broad range, from tens of angstroms to hundreds of angstroms or more. It should also be noted that the particles of the semiconducting phase are in a liquid state during the heat treatment, since the melting point of the CuCl crystals is 440°C, and these crystals are approximately spherical in shape because of the surface tension. It may be that the particles retain this shape during crystallization and that the microscopic CuCl crystals in the matrix can be assumed spherical, in a first approximation.
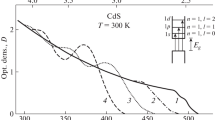
Figure 2 shows the absorption spectra measured at T = 4.2 К for three samples, differing in the average radius of the microscopic crystals. These spectra were recorded with a dual-beam Perkin-Elmer Model 555 spectrometer with samples d = 0.1 mm in thickness. Such samples could be used because of the relatively low concentration of the crystalline phase in the glass. As can be seen in Fig. 2, at sufficiently large values of the average radius (\(\bar {a}\) = 310 Å) the absorption spectrum of the microscopic CuCl crystals dispersed in the matrix has two intense lines (λ = 3785 and 865 Å), which result from the excitation of excitons associated with two spin- orbit-split valence subbands. This spectrum is the same as the absorption spectrum of thin CuCl films [6]. As the average radius of the microscopic crystals is reduced, we observed a significant short-wave shift and a broadening of the exciton-absorption lines remaining in the spectrum, down to the smallest crystal sizes studied, \(\bar {a}\) = 20 Å.
The observed dependence of the spectral position of the exciton absorption lines on the microscopic crystals may be a consequence of a quantum size effect [7]. The current carriers and excitons in a semiconducting crystal in a dielectric matrix are trapped in a potential well whose walls are the boundaries of the microscopic crystal. As the size of the crystal is reduced, the energy of the particles in the potential well increases, and this increase can lead to the observed short-wave shift of the absorption lines. Under the assumption of a spherically symmetric potential well of infinite depth, and if the size dispersion of the particles is neglected, the short-wave shift resulting from the size quantization of a particle of mass m can be described by [7]
As can be seen from the experimental dependence of the spectral position of the exciton absorption lines on the average radius of the microscopic crystals (Fig. 3), the short-wave shift is indeed a linear function of l/\({{\bar {a}}^{2}}\) over a broad range of sizes. The effective mass found from the slope of the lines in Fig. 3 and Eq. (2) is m = 1.2m0, where m0 is the mass of the free electron. This effective mass is at odds with values given in the literature for the effective masses of electrons (me = 0.44m0) and holes (mh = 3.6m0), and it is therefore at odds with the reduced and translational masses of excitons in CuCl crystals [8]. The discrepancy may be caused primarily by the size dispersion of the particles, which must be taken into account for measuring the radius of the microscopic crystals by the X-ray scattering method and also in determining the effective mass from the dependence of the short-wave shift on 1/\({{\bar {a}}^{2}}\).
REFERENCES
R. Dingle, in Festkorperprobleme, Vol. 15 of Advances in Solid State Physics (Pergamon/Vieweg, Braunschweig, 1975), p. 21.
A. Hartstein and A. Fowler, in Proceedings of the 13th International Conference on the Physics of Semiconductors, Rome, 1976, p. 741.
A. I. Ekimov, A. A. Onushchenko, and V. A. Tsekhomskii, Fiz. Khim. Stekla 6, 511 (1980).
I. M. Lifshitz and V. V. Slezov, Sov. Phys. JETP 8, 331 (1959).
V. V. Golubkov, A. P. Titov, and E. A. Porai-Koshits, Prib. Tekh. Eksp., No. 1, 215 (1975).
S. Nikitine, in Progress in Semiconductors (Heywood, London, 1962), Vol. 6, p. 269.
A. S. Davydov, Quantum Mechanics (Nauka, Moscow, 1963; Pergamon, New York, 1965).
N. Kato, T. Goto, T. Fujii, and M. Ueta, J. Phys. Soc. Jpn. 36, 169 (1974).
ACKNOWLEDGMENTS
We wish to thank V.I. Safarov for useful discussions and V.A. Tsekhomskii for furnishing the glass samples. We wish to thank V.V. Golubkov for carrying out the X-ray measurements.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by Dave Parsons
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ekimov, A.I., Onushchenko, A.A. Quantum Size Effect in Three-Dimensional Microscopic Semiconductor Crystals. Jetp Lett. 118 (Suppl 1), S15–S17 (2023). https://doi.org/10.1134/S0021364023130040
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364023130040