Abstract—
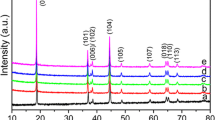
The stability of the LiCoPO4 electrode material in contact with a NASICON-type lithium ion conducting solid electrolyte having the composition Li1.5Al0.5Ge1.5(PO4)3 (LAGP) and a wide range of electrochemical stability has been studied for the first time. We have found the optimal temperature at which there is no reaction between the starting components of the composites and they retain their chemical and thermal stability. Cosintering a mechanical mixture of synthesized single-phase LiCoPO4 and LAGP powders with a submicron particle size at 750°C, we have obtained a stable LiCoPO4/LAGP two-phase composite whose room-temperature electrical conductivity is three orders of magnitude higher than that of the lithium cobalt double phosphate. Composites based on LiCoPO4 and the LAGP ionic conductor can be regarded as promising cathode materials for all-solid-state lithium ion batteries.





Similar content being viewed by others
REFERENCES
Kulova, T.L. and Skundin, A.M., High-voltage materials for positive electrodes of lithium ion batteries (review), Russ. J. Electrochem., 2016, vol. 52, no. 6, pp. 501–524.
Prabu, M., Selvasekarapandian, S., Kulkarni, A.R., et al., Ionic transport properties of LiCoPO4 cathode material, Solid State Sci., 2011, vol. 13, pp. 1714–1718.
Harada, R., Aso, K., Hayashi, A., and Tatsumisago, M., Preparation of composites with LiCoPO4 electrode and LiTi2(PO4)3 electrolyte for bulk-type all-solid-state lithium batteries, Electrochemistry, 2015, vol. 83, no. 10, pp. 898–901.
Allen, J.L., Thompson, T., Sakamoto, J., et al., Transport properties of LiCoPO4 and Fe-substituted LiCoPO4, J. Power Sources, 2014, vol. 254, pp. 204–208.
Svitan’ko, A., Scopets, V., Novikova, S., and Yaroslavtsev, A., The effect of composite formation with oxides on the ion conductivity of NASICON-type LiTi2(PO4)3 and olivine-type LiFePO4, Solid State Ionics, 2015, vol. 271, pp. 42–47.
Svitan’ko, A.I., Novikova, S.A., Kulova, T.L., Skundin, A.M., and Yaroslavtsev, A.B., An improvement in the ionic conductivity and electrochemical characteristics of LiFePO4 by heterogeneous doping with NASICON-type phosphate, Mendeleev Commun., 2015, vol. 25, pp. 207–208.
Ren, Y., Chen, K., Chen, R., et al., Oxide electrolytes for lithium batteries, J. Am. Ceram. Soc., 2015, vol. 98, pp. 3603–3623.
Jian, Z., Hu, Y.-S., Ji, X., and Chen, W., NASICON-structured materials for energy storage, Adv. Mater., 2017, vol. 29, no. 20, paper 1 601 925.
Deiner, L.J., Howell, T.G., Koenig, G.M., and Rottmayer, M.A., Interfacial reaction during co-sintering of lithium manganese nickel oxide and lithium aluminum germanium phosphate, Int. J. Appl. Ceram. Technol., 2019, vol. 16, pp. 1659–1667.
Nagata, K. and Nanno, T., All solid battery with phosphate compounds made through sintering process, J. Power Sources, 2007, vol. 174, pp. 832–837.
Gellert, M., Dashjav, E., Grüner, D., et al., Compatibility study of oxide and olivine cathode materials with lithium aluminum titanium phosphate, Ionics, 2018, vol. 24, no. 4, pp. 1001–1006. https://doi.org/10.1007/s11581-017-2276-6
Yu, S., Mertens, A., Tempel, H., Schierholz, R., Kungl, H., and Eichel, R.-A., Monolithic all-phosphate solid-state lithium-ion battery with improved interfacial compatibility, ACS Appl. Mater. Interfaces, 2018, vol. 10, pp. 22 264–22 277.
Okumura, T., Takeuchi, T., and Kobayashi, H., Application of LiCoPO4 positive electrode material in all-solid-state lithium-ion battery, Electrochemistry, 2014, vol. 82, no. 10, pp. 906–908.
Kunshina, G.B., Ivanenko, V.I., Gromov, O.G., and Lokshin, E.P., Production of an electrode material modified by a lithium-conducting solid electrolyte, Russ. J. Inorg. Chem., 2014, vol. 59, no. 10, pp. 1175–1179.
Feng, J.K., Lu, L., and Lai, M.O., Lithium storage capability of lithium ion conductor Li1.5Al0.5Ge1.5(PO4)3, J. Alloys Compd., 2010, vol. 501, pp. 255–258.
Liu, Y., Chen, J., and Gao, J., Preparation and chemical compatibility of lithium aluminum germanium phosphate solid electrolyte, Solid State Ionics, 2018, vol. 318, pp. 27–34.
Delaizir, G., Viallet, V., Aboulaich, A., et al., The stone age revisited: building a monolithic inorganic lithium-ion battery, Adv. Funct. Mater., 2012, vol. 22, pp. 2140–2147.
Aboulaich, A., Bouchet, R., Delaizir, G., et al., A new approach to develop safe all-inorganic monolithic Li-ion batteries, Adv. Energy Mater., 2011, vol. 1, pp. 179–183.
Kubanska, A., Castro, L., Tortet, L., et al., Effect of composite electrode thickness on the electrochemical performances of all-solid-state Li-ion batteries, J. Electroceram., 2017, vol. 38, nos. 2–4, pp. 189–196.
Robinson, J.P., Kichambare, P.D., Deiner, J.L., et al., High temperature electrode–electrolyte interface formation between LiMn1.5Ni0.5O4 and Li1.4Al0.4Ge1.6(PO4)3, J. Am. Ceram. Soc., 2018, vol. 101, no. 3, pp. 1087–1094.
Kunshina, G.B., Bocharova, I.V., and Lokshin, E.P., Synthesis and conductivity studies of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte, Inorg. Mater., 2016, vol. 52, no. 3, pp. 279–284.
Kunshina, G.B., Bocharova, I.V., and Ivanenko, V.I., Preparation of the Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte with high ionic conductivity, Inorg. Mater.: Appl. Res., 2017, vol. 8, no. 2, pp. 238–244.
Tananaev, I.V. and Avduevskaya, K.A., Phase relations in the GeO2–H2C2O4–H2O system at 25°C, Zh. Neorg. Khim., 1958, vol. 3, no. 9, pp. 2172–2177.
Ivanenko, V.I., Aksenova, S.V., Lokshin, E.P., et al., Synthesis of phase-pure transition metal double phosphate nanopowders, Tr. Kol’sk. Nauchn. Tsentra Ross. Akad. Nauk, 2015, no. 31, pp. 354–358.
Gnedenkov, S.V. and Sinebryukhov, S.L., Impedance spectroscopy in studies of charge transport processes, Vestn. Dal’nevostochn. Otd. Ross. Akad. Nauk, 2006, no. 5, pp. 6–16.
Rissouli, K., Benkhouja, K., Ramos-Barrado, J.R., and Julien, C., Electrical conductivity in lithium orthophosphates, Mater. Sci. Eng., B, 2003, vol. 98, no. 3, pp. 185–189.
Funding
This research was supported in part by the Presidium of the Russian Academy of Sciences, program no. 22: Promising Physicochemical Technologies for Specialty Applications.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kunshina, G.B., Bocharova, I.V. & Ivanenko, V.I. Compatibility of LiCoPO4 Cathode Material with Li1.5Al0.5Ge1.5(PO4)3 Lithium-Ion-Conducting Solid Electrolyte. Inorg Mater 56, 204–210 (2020). https://doi.org/10.1134/S0020168520020089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168520020089