Abstract
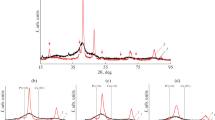
Nanostructured Pt/C electrocatalysts containing about 20 wt % Pt have been produced by chemical reduction in Pt(IV) solutions. The nature of the reductant (sodium borohydride, ethylene glycol, formaldehyde, or formic acid) and the associated changes in synthesis conditions have a significant effect on the structural characteristics of the materials obtained. In particular, the average size of Pt nanoparticles (crystallites) ranges from 1.8 to 5.5 nm. The largest electrochemically active surface area of the Pt in the catalysts obtained in this study (128 m2/g Pt) considerably exceeds that of E-TEK, a commercially available Pt/C catalyst similar in composition ( 110 m2/g Pt).
Similar content being viewed by others
References
Gasteiger, H.A., Kocha, S.S., Sompalli, B., et al., Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs, Appl. Catal. B, 2005, vol. 56, pp. 9–35.
Shao-Horn, Y., Sheng, W.C., Chen, S., et al., Instability of supported platinum nanoparticles in low-temperature fuel cells, Top. Catal., 2007, vol. 46, pp. 285–305.
Yaroslavtsev, A.B., Dobrovol’skii, Yu.A., Shaglaeva, N.S., et al., Nanostructured materials for low-temperature fuel cells, Usp. Khim., 2012, vol. 81, pp. 191–220.
Tarasevich, M.R., State-of-the-art cathode catalysts and related fuel cells, Al’tern. Energ. Ekol., 2010, vol. 85, no. 5, pp. 135–137.
Zhang, J., Wang, X., Wu, C., et al., Preparation and characterization of Pt/C catalysts for PEMFC cathode: effect of different reduction methods, React. Kinet. Catal. Lett., 2004, vol. 83, no. 2, pp. 229–236.
Chen, J., Jiang, C., Yang, X., et al., Studies on how to obtain the best catalytic activity of Pt/C catalyst by three reduction routes for methanol electro-oxidation, Electrochem. Commun., 2011, vol. 13, pp. 314–316.
Ma, H-C., Xue, X-Z., Liao, J-H., et al., Effect of borohydride as reducing agent on the structures and electrochemical properties of Pt/C catalyst, Appl. Surf. Sci., 2006, vol. 252, pp. 8593–8597.
Prabhuram, J., Zhao, T.S., Wong, C.W., et al., Synthesis and physical/electrochemical characterization of Pt/C nanocatalyst for polymer electrolyte fuel cells, J. Power Sources, 2004, vol. 134, pp. 1–6.
Nores-Pondala, F.J., Vilellab, I.M., Troianic, H., et al., Catalytic activity vs. size correlation in platinum catalysts of PEM fuel cells prepared on carbon black by different methods, Int. J. Hydrogen Energy, 2009, vol. 34, pp. 8193–8203.
Qi, J., Jiang, L.H., Jing, M.Y., et al., Preparation of Pt/C via a polyol process—investigation on carbon support adding sequence, Int. J. Hydrogen Energy, 2011, vol. 36, pp. 10 490–10 501.
Favilla, P.C., Acosta, J.J., Schvezov, C.E., et al., Size control of carbon-supported platinum nanoparticles made using polyol method for low temperature fuel cells, Chem. Eng. Sci., 2013, vol. 101, pp. 27–34.
Guterman, V.E., Pakharev, A.Y., Tabachkova, N.Y., et al., Microstructure and size effects in Pt/C and Pt3Ni/C electrocatalysts synthesised in solutions based on binary organic solvents, Appl. Catal., A, 2013, vol. 453, pp. 113–120.
Guterman, V.E., Belenov, S.V., Dymnikova, O.V., et al., Influence of water-organic solvent composition on composition and structure of Pt/C and PtxNi/C electrocatalysts in borohydride synthesis, Inorg. Mater., 2009, vol. 45, no. 5, pp. 498–505.
Pabst, W. and Gregorová, E., Characterization of Particles and Particle Systems, Prague: ICT, 2007.
Guterman, V.E., Lastovina, T.A., Belenov, S.V., et al., PtM/C (M = Ni, Cu, or Ag) electrocatalysts: effects of alloying components on morphology and electrochemically active surface areas, J. Solid State Electrochem., 2014, vol. 18, pp. 1307–1317.
Gusev, A.I., Nanomaterialy, nanostruktury, nanotekhnologii (Nanomaterials, Nanostructures, and Nanotechnologies), Moscow: Fizmatlit, 2009.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.A. Alekseenko, V.E. Guterman, V.A. Volochaev, S.V. Belenov, 2015, published in Neorganicheskie Materialy, 2015, Vol. 51, No. 12, pp. 1355–1360
Rights and permissions
About this article
Cite this article
Alekseenko, A.A., Guterman, V.E., Volochaev, V.A. et al. Effect of wet synthesis conditions on the microstructure and active surface area of Pt/C catalysts. Inorg Mater 51, 1258–1263 (2015). https://doi.org/10.1134/S0020168515120018
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168515120018