Abstract
The current study was focused on Fe oxyhydroxides from the north-western part of the East-European platform. Modern-day Fe oxyhydroxides of bacterial origin demonstrate an enhanced concentration of rare earth elements (up to 1200 ppm), compared to samples without iron bacteria. The 143Nd/144Nd ratio in bacterial Fe oxyhydroxide has the value from 0.511532 to 0.512588 and corresponds to the geochemical signature of the waters, oxyhydroxides precipitated from. Samples of iron hydroxides from Quaternary and recent continental ore deposits with different Nd and Sr isotope composition were used for the laboratory reduction of Fe3+ up to emergence of magnetite (T ~ 1000°C). 143Nd/144Nd and 87Sr/86Sr ratios in the newly formed mineral phases show insignificant discrepancy with parent iron ore. The persistency of Sm–Nd and Rb–Sr isotope systems in the process of bog iron ore experimental melting permits it’s applying to paleoenvironment reconstructions and archaeometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Redox-sensitive oxidation of Fe and its presence on the Earth surface in Fe(II) and Fe(III) forms allows using Fe-bearing minerals as a substrate for the reconstruction of redox conditions in the past. Banded iron formations (BIFs) are among the most studied iron-bearing geological formations, were hematite, magnetite and other iron-oxyhydroxides are considered as proxies of the Precambrian oceans (Schad et al., 2022; Dodd et al., 2022). Isotopic data on iron, Nd and Sr, obtained for BIFs in last decades (Frei et al., 1999; Halverson et al., 2011; Li et al., 2015; Viehmann et al., 2015; Haugaard et al., 2016; Konhauser et al., 2017; Alfimova et al., 2019) significantly improved our understanding of BIF sedimentation, as a chemical sediment, deposited directly from the seawater.
The role of organic matter in this process is still discussed (Dodd et al., 2022; Konhauser et al., 2017). One of the first ones, F. Chukhov pointed to potential role of iron-oxidizing bacteria in the process of Fe precipitation in fresh water and paralic facies with forming of primary bacterial ferrihydrite (Chukhrov et al., 1973, 1975) and suggested the possible bacterial origin of hematite pigment in red beds and banded iron formations. Metabolism of informal group of iron-oxidizing bacteria is based on iron oxidizing and these bacteria play an important role in modern iron cycle (Emerson et al., 2010).
Iron-bearing minerals, formed after bacterial ferrihydrite, have been reported to have a very high (up to hundreds ppm) content of trace elements, including rare earth elements (REE). This characteristic is explained by the very high sorption capacity of bacterial ferrihydrites (Andersson and Pedersen, 2003; Kurz et al., 2011). At the same time iron minerals of magmatic origin do not show such high concentrations of REEs (Rhodes et al., 1999; Selmi et al., 2009; Zamanian and Radmard, 2016). Such a particularity of bacterial oxyhydroxides might have an important outcome—high content of REEs might be indicating the bacterial origin of iron oxyhydroxides and furthermore be useful for the reconstruction of provenance and geochemical palaeofacies.
East European Platform is the area with a lot of modern springs enriched with Fe(II), numerous Q-R sites of bog ores, even the first iron-mineral water resort in Russia was founded here (Felitsyn and Bogomolov 2016, 2017; Svetov and Svetova, 2021), so is a good place for study of iron oxides which can be of bacterial or abiogenic origin, affected neither by diagenesis, nor metamorphism.
On the north-western part of the East European Platform (EEP) modern iron bacteria occur in many springs (Felitsyn and Alfimova, 2017). Geochemical study of the bacterial induced iron oxyhydroxides from those springs can be very useful for estimating the sorption capacity of the latter ones. Modern iron bacteria also occur in corroded parts of modern Fe-based alloys, though the geochemical trace of bacteria in that process was not previously estimated. To find bacterial indicators and to reduce the influence of diagenetic processes on the geochemical signatures, it is worth studying the chemical isotopic composition of the modern iron alloy and iron oxide rust formed on it, coupled with microbiological identification of bacterial communities, which may or may not have influenced the oxidation process.
In the current manuscript, we present data on trace elements and the isotopic composition of Nd and Sr from iron artifacts of different ages (from modern ones to ones which are several hundred years old) and recent bacterial communities with iron-oxidizing bacteria, collected from the North-Western part of the EEP.
Iron-rich geological formations, as all other geological objects and Precambrian especially, have undergone diagenesis and often, metamorphism. After burial trace elements and isotopic signature in Fe oxyhydroxide could be modified and thus, special attention should be paid to the possible effects of high temperature influence to the stability of geochemical and isotopic signatures, when dealing with highly reactive minerals, such as oxides and hydroxides. We conducted laboratory experiment with modern day iron oxides, which is also described in this manuscript. The specific goal of the experiment was the comparison of Nd and Sr isotope signatures in Quaternary and recent continental iron deposits (composed of limonite and hydrogoethite) and magnetite obtained from these ores through reduction with charcoal in the laboratory condition and thus, estimating the stability of the isotopic system through metamorphic changes.
SAMPLES ORIGIN
We examined rust–covered iron-made items of different ages and air-dry material containing Fe oxyhydroxide from bacterial communities with iron-oxidizing bacteria from the north-western part of EEP. Here, we use the term “Fe oxyhydroxide” as a complex term for a mixture of goethite, hydro-goethite and other Fe compounds of the hydroxide group and water, which we found in bacterial mats.
The Q-R continental iron ore samples used in laboratory experiment were taken from the sites of the Baltic craton and East European platform, where bog iron ore has been explored for the manufacturing of red pigment—Polovinkino deposits (the Baltic craton, Pryazha region, Southern Karelia, Russian Federation) and Somino deposit (the East-European platform, Somino settlement, Saint-Petersburg region, Russian Federation). In both deposits the productive layer (~1.0 m thick) is located between the Quaternary lacustrine-glacial sediments and soil cover and consists of hydrogoethite, goethite and small amount of vivianite and psilomelane. The content of Fe in both deposits varies from 31.4 to 35.7 wt % and H2O—from 5.2 to 10.9 wt %; a detailed description of elemental, mineralogical and isotopic composition in Polovinkino and Somino iron ores is presented in (Felitsyn and Bogomolov, 2016).
Hydrogoethite originating from primary bacterial ferrihydrite in the bacterial mats at sites of groundwater seepage on the right bank of the Sestra Zavodskaya River near the town of Sestroretsk (~30 km northwest of Saint-Petersburg, Russian Federation) was also studied. The yellow-brown gel-like bacterial mat is composed of characteristic twisted stalks coated by iron hydroxides, identified as the ferrobacteria Gallionella spp. An average Fe content in such ferrobacteria from the Sestra Zavodskaya River is 37.9 wt %; data on composition of the studied bacterial iron hydroxides are in (Felitsyn and Alfimova, 2017). The third location for modern day bacterial mat sampling was the spring on the south-western edge of Lake Orlinskoye, ~ 90 km south of Saint-Petersburg, in the vicinity of the village of Zaozerje.
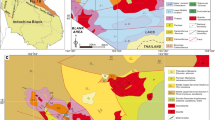
The iron items included in this study are of two origins—recently produced iron alloys, and archeological artifacts (Table 1, Fig. 1). The archeological artifacts studied are divided into two parts: nails from wooden planks found in old buildings, and iron-made artifacts from soil horizons (0–20 cm deep). These objects had been chosen to characterize different preservation conditions.
The locations of studied samples on the north-western part of the East European Platform. Numbers correspond to sample number in Table 1.
Metallic constructions on the right bank of Sestra Zavodskaya river (samples #9m, #9h and #10m, #10h) were built in the 1980’s near the spring to prevent the slope collapse. The pH of the ground water in this spring varies from 6.6 to 7.1. For each item, chemical analysis of the inner metal part and the rust cover was provided. The rust cover is 1.0–3.0 mm thick and is composed of goethite and hydrogoethite.
ANALYTICAL TECHNIQUES
Geochemical Studies
Isotopic composition of samples was measured in laboratory of IPGG RAS, chemical composition was done in VSEGEI. Prior to any isotopic studies all the samples were dissolved in a warm, weak HCl (T = 40°C) solution, in order to avoid possible contamination with heavy minerals (garnet, zircon, titanite, etc.). Obtained by such procedure, an aliquot of sample with iron oxides was dried, and only after that dissolved in HF.
For isotopic analysis, all samples were completely dissolved in a mixture of double distilled 20N HF and double distilled 3N HCl (1 : 3 proportion), then the solutions were centrifuged to obtain a clear solution (3 mL), to use for cation-exchange chromatography.
The concentrations of the Sm, Nd, Rb and Sr were determined by isotope dilution using mixed 149Sm–150Nd and 85Rb–84Sr spikes. The separation of Sm and Nd was accomplished using two stages of column chromatography. The first stage separated REEs from the bulk of the sample using cation exchange chromatography with AG50W-X8 resin. The second stage included extraction chromatography using a liquid HDEHP as a cation exchange medium. The Sm–Nd and Rb–Sr isotopes in the samples were analyzed using a standard procedure in a multi-collector TRITON mass-spectrometer (Thermo Fisher Scientific, Germany) operating in a static mode. A correction for Nd isotope fractionation was introduced using the normalization of the measured values to 146Nd/144Nd = 0.7219. The normalized ratios were adjusted to 143Nd/144Nd = 0.511860 in the La Jolla Nd isotopic standard. A correction for Sr isotope fractionation was introduced using the normalization of the measured values to 88Sr/86Sr = 8.37521. The errors of the determination of Sm, Nd, Rb and Sr concentrations were 0.5%. The Sm and Nd blanks were 10 and 20 pg, respectively. The Rb and Sr blanks were each 30 pg. Replicate analyses of the BCR-2 standard yielded 6.57 ± 0.01 (6.58 ± 0.04) ppm Sm, 28.6 ± 0.01 (28.7 ± 0.02) ppm Nd, 147Sm/144Nd = 0.1388 ± 0.003 (0.1388 ± 0.003), and 143Nd/144Nd = 0.512627 ± 0.000001 (0.512630 ± 0.000001); 46.9 ± 0.08 (46.9 ± 0.02) ppm Rb, 340 ± 0.006 (340 ± 0.001) ppm Sr, 87Rb/86Sr = 0.3986 ± 0.0008 (0.3990 ± 0.0009), and 87Sr/86Sr = 0.704966 ± 0.000012 (0.704960 ± 0.000016) (mean of 25 measurements). The values for BCR-2 are given in parentheses.
Element concentration was measured by Agilent 7500c ICP-MS (USA), the average accuracy in REE measurements is within 7%. A scanning electron microscope JSM-6510LA (Japan) equipped with a JED-2200 (JEOL) energy dispersive spectrometer was used for the analysis of objects and obtaining images.
Laboratory Melting of Iron Ore
As one of the goals of current study was the estimation of isotope fractionation in the iron ore during the experiment, we need the data on charcoal, used as a reducing agent. The criterion for charcoal selection was its Sr and Nd isotope composition—different from ones in the studied iron hydroxides located at the Baltic craton and on the margin of the East-European platform. For this purpose, the charcoal manufactured from the timber wood of Betula pendula, grown in another geochemical province (central part of the East-European platform, Kostroma region, 300 km northeast of Moscow, Russian Federation) was used.
All samples of iron ore represent a fine-grained substance (particle size from 20 to 100 μm) because the extraction of iron minerals was performed by means of hydro gravitational method and terminal cleaning by an electric magnet with optic microscopy control without preliminary crushing. These fractions of iron oxyhydroxide were used for reduction.
The reduction of Fe(III)-containing fractions of iron oxyhydroxides occurred under the introduction of charcoal as a reductant at the temperature of 950°С according to the following scheme: Fe2O3 + 3C → 2Fe + 3CO↑. The excess charcoal, 3.15 g, which is 7 times higher than the stoichiometric calculated mass, was milled to a powdery state in an agate mortar. Then the charcoal powder was placed in a platinum crucible. A layer of iron ore (2 g) was added to cover the underneath layer of charcoal. The crucible was covered leaky with a platinum plate to create a lack of oxygen during calcination which results in carbon monoxide generation and incomplete burnout of the charcoal. Calcination underwent in a high temperature electrical oven TZ-1700 mini (Thermoceramics Ltd., Russian Federation) equipped with a microprocessor regulator of temperature. The rate of heating was approximately 5°С per a minute. The program involved two steps of heating (up to 350 and to 950°С) and two steps of exposure (at 350°С—10 min and at 950°С—2.5 h). The chemical reaction resulted in the formation of incompletely burned charcoal and new mineral phases. The obtained material was carefully purified from charcoal and slag particles with magnets and examined using an electronic microscope JSM-6510LA (Japan) equipped with a spectrometer JED-2200 (JEOL). The reduced substance was identified as cryptocrystalline magnetite with inclusion of rounded metal iron particles up to 10 μm in diameter (Fig. 2). The charcoal sample (ca. 10 g) was converted to a soluble form with a mixture (3 : 1) of 37% HCl and 79% HNO3 within 5 days at 100°C for the determination of Sr and Nd isotopes composition
Microbiological Studies
Detailed microbiological studies were done in the Institute Pasteur, Saint-Petersburg for five rust samples, including modern day iron items and biofilms with iron oxidizing bacteria from the spring on the right bank of Sestra Zavodskaya river (samples #9b, 10b), and rust on archeological artifacts from the top-soil horizon (samples #2 and #3, Table 1).
The bacteriological studies involved the adjusted bacteriological method of iron-bacteria excretion with the help of nutrients, light microscopy with and without sample coloring, scanning electron microscopy and mass-spectrometry (MALDI-TOFF). The studies were conducted in Institute Pasteur (Saint-Petersburg) by L.A. Kraeva.
Nutrients for growing iron-bacteria are based on the mixture of agar-agar by Givental-Vedmina (AGV) with 3.95 g of Mohr’s salt and 1.6 g of purified sodium hydroxide. The nutrient preparation method with Fe(II) was used.
The sodium hydroxide was dissolved in 50 mL of sterilized distilled water. The Mohr’s salt was heated up to 1000°C until the substrate started discoloring and condensing. Accounting for weight loss of salt, 40 mL of water had been used further. When the aforementioned ingredients were mixed, the following solution with sediments was obtained:
The liquid part was extracted and the sediment was mixed with 200 mL of hot AGV, poured into the Petri dishes, and cooled. Special care was taken to rapidly prepare the nutrient, because Fe2+ can be rapidly oxidized to Fe3+ and change color.
After the nutrients were cooled and dried, the sowing of material (bacteria) was done. Then it was left for 24 h in microaerophilic conditions and T = 28°С, then in standard conditions for 3–5 days until the bacteria colonies could be seen clearly. After that, the “clear” iron bacteria were obtained from every type of colony which appeared, and the amount of bacteria was calculated in 1 mL of the sample.
The proliferation of iron bacteria was recorded in the experiment on the nutrient with Fe2+ concentration over 7.5 mg/g. In examples with lower concentrations of iron, no bacterial growth was detected. Microscopic studies were carried out with an Axio Scope A1 Zeiss microscope for the initial sample and for the grown bacteria strain, both with and without coloring. Mass-spectrometry for microbiological investigations was done with the MicroflexTM LT MALDI-TOF (Bruker Daltonics, Germany), Flex control program with linear positive regime. Spectral interpretation was done with MALDI Biotyper 3.0 (Bruker Daltonics) software. Taxonomical identification is based on Coincidence value (SV): SV > 2.3 means reliable identification of species, 2.66 < SV < 2.00—reliable identification of genus, 1.999 < SV < 1.7—probable genus identification, SV < 1.7—unreliable result.
RESULTS
Iron-made Artifacts of Different Ages
REE patterns in the rust cover of an artifact and the metallic part are almost equal (Fig. 3, Table 2). At the same time, Ce concentrations in all types of the studied artifacts display significant differences: all oxidized crusts are enriched with Ce, compared to La and Pr, whereas in metallic Fe, a negative Ce anomaly is observed. Oxidizing of Fe0 does not lead to Eu partitioning: a positive Eu anomaly is observed both in rust cover on the nails from the roof of the Stock Exchange building and their inner part (samples #5 and #6). At the same time negative Eu anomaly is observed both in the outer and inner parts of the bracket from the village of Tolmachevo (sample #2). In general, REE patterns in rust cover are similar to those in parent metallic iron, whereas the REE concentration in Fe oxyhydroxide crust is 5–10 times in magnitude higher than in the metallic part of the iron artifacts.
Shale–normalized rare earth elements patterns for studied artifacts without iron-oxidizing bacteria in rust cover. Solid line-metallic inner part, dashed line—rust cover. Sample numbers correspond to those in Table 1. Concentrations of REE in NASC after Gromet et al. (1984).
The concentration of Fe in rust varies from a weight percentage of 33 to 70. In the inner parts of the artifacts the weight percentage Fe is 91–97 wt %. Rust cover is significantly depleted of Mn, compared with metallic Fe parts. Maximum depletion (60 times) is reported in the oxidized cover of the arrowhead from the village of Krechevitsy (sample #4).
Aluminum content is within 2240–4075 ppm for the inner part of the artifacts, and varies from 77 to 167 ppm in rust, an average concentration of Zr is 3.1 ± 1.7 ppm in the inner parts, and 0.98 ± 0.44 ppm in Fe-oxides on the surface of artifacts. Rust cover contains less Ti and more P, compared to the inner metallic part of the artifacts. No significant difference is observed in Ca and S concentrations (Table 2).
In modern-day iron items, there is also difference in Mn concentration in the metallic part, and in rust cover: the inner parts contain around 1 wt % of Mn, whereas oxide cover—0.1–0.4 wt % (SEM data).
The isotopic composition of Nd is roughly the same in the inner parts of the artifacts and in rusted ones. However, Sr isotopic composition as well as its concentration is drastically different (Table 3, Fig. 4). The 87Sr/86Sr ratio is much smaller in the rust than in metallic iron, and Δ87Sr/86Sr varies from 0.022925 for sample #7, Murino church artifacts to 0.005465 for sample #6 (square headed nails from the Stock Exchange building). Taking into account the known dates of the buildings’ construction (see samples description), the rate of 87Sr/86Sr changes can be estimated as 0.00003 from 87Sr/86Sr ratio per year for squareheaded nails from the Stock Exchange building, 0.00006 for the roundheaded nail from the same building, 0.00008 for the Solovky nail, and 0.00010 for the Murino church nail.
Variations in Nd and Sr isotope ratios in iron items. Metallic iron—filled symbols, rust cover—open symbols. Samples without iron bacteria: circle—sample #7, square—sample #8, triangle—sample #5, diamond—sample #6; samples with iron bacteria (encircled symbols): asterisk—sample #9, hexagon—sample #10.
The isotopic composition of Nd and Sr in metallic iron and rust cover from modern day iron items near the Sestra Zavodskaya river (samples #9, 10) demonstrates an other pattern—in these pairs, both 87Sr/86Sr and 143Nd/144Nd ratios in rust cover are significantly different from the parental metallic iron. It is noteworthy that the 87Sr/86Sr ratio in rust from pitting on the pipe (sample #9) is significantly lower than in the parent iron alloy, whereas in rust from the lath wire (sample #10), the proportion of radiogenic Sr is higher than in the metallic substrate. The values of the 143Nd/144Nd ratio in rust are substantially lower than in the metallic part in both cases. The rate of 87Sr/86Sr ratio modification during Fe0 oxidization is 0.00050 per year for sample #10 and 0.000368 per year for sample #9. The rate of 143Nd/144Nd ratio values transformation is 0.000016 and 0.000020 respectively.
Bacterial Communities from the Northwestern Part of EEP
Iron oxidizing bacteria constitute a minor part of the active forms in bacterial communities: even in biofilms they do not exceed 3%, in the rust of sample #10b, fully composed of Gallionella ferruginea remnants, they comprise around 20% and in sample #9b iron bacteria hardly exceed 0.3% (Table 4, Fig. 5). The concentration of these bacteria was 2 × 103–8 × 104 colony forming units/ml, which is typical for iron rich environment.
A SEM study of modern-day iron products from the Sestra Zavodskaya river vicinity showed that the rust cover on sample #10 is mainly composed of iron bacteria remnants. A microbiological study revealed a variety of iron- oxidizing bacteria in the same sample, with Gallionella ferruginea as the dominant form. In the Fe oxyhydroxides from sample #9 Leptothrix ochracea is dominant (Table 4).
The occurrence of Pseudomonas fluorescens in the studied samples, combined with the absence of Bacillus cereus in them, points to the possibility of phyto- and bioremediation at the places where the samples were collected. The full list of discovered iron bacteria can be found in Table 4.
Data on the isotopic Sm–Nd system in bacterial mats from the spring on the right bank of Sestra Zavodskaya river and near the Lake Orlinskoye (Table 5), show 143Nd/144Nd around 0.5115–0.5118. These values are characteristic for iron ores originating from the northwestern part of EEP (Felitsyn et al., 2019). At the same time, the concentrations of Sm and Nd in these samples is double the amount found in NASC. The enrichment factor (NASC) compared with standard shale for iron artifacts without iron- oxidizing bacteria is 3.8–6.4 for Nd, for Sm 3.8–8.0. For modern day iron items with iron-oxidizing bacteria (samples #9, 10) the enrichment factor for Nd is 105–160, for Sm 122–167.
Thus, all studied iron oxides and hydroxides can be divided into two groups—(1) those with high, elevated concentrations of Sm and Nd, and (2) those with low concentrations of these elements. Samples which are placed in the first and the second group, do not show any connection with the age of the sample or its location, but samples from the group 1 do show the presence of iron-oxidizing bacteria according to microbiological studies, while samples from group 2 do not (Table 5).
Laboratory Melting of Iron Ore
Concentrations of Sm, Nd, Rb and Sr are significantly different in studied continental Fe3+-bearing oxide minerals and vary from n × 0.1 to n × 100 ppm for Nd and from n × 10 to n × 100 ppm for Sr (Table 6). Fe oxyhydroxide formed on ferrihydrite in bacterial mats with ferrobacteria Gallionella spp. from Sestroretsk displays the maximal content of rare earth elements— up to ~1200 ppm, according to previous studies (Felitsyn and Alfimova, 2017). Concentrations of Sm and Nd in charcoal are close to these in iron ore from Polovinkino deposit; Rb and Sr content has the same order of magnitude as iron ore from Somino deposit.
The enrichment of reduced magnetite and metal iron with all measured elements in comparison with parent iron ore is evident. Enrichment factor reaches ~4 for Sm in Somino samples pair and ~20 for Rb in Sestroretsk samples pair. Charcoal has minimal 143Nd/144Nd and 87Sr/86Sr isotopic ratios in the studied samples. Nd isotope composition in Fe oxyhydroxide varies from a relatively high radiogenic 143Nd content in Somino ore to the lowest in Sestroretsk bacterial mats (Table 6). The low 87Sr/86Sr ratio is typical for Somino ores. Fe oxyhydroxide from Polovinkino ore deposit at the Baltic craton displays maximal enrichment in radiogenic 87Sr. A distinctive shift in Sr and Nd isotope composition between parent ore and magnetite was revealed—in all Fe oxyhydroxide-magnetite pairs 143Nd/144Nd and 87Sr/86Sr ratios in magnetite are lower than in the Fe oxyhydroxide source (Fig. 6). The maximum discrepancy for Nd isotope system (Δ143Nd/144Nd = 0.000021) detected for Polovinkino Fe oxyhydroxide-magnetite couple, whereas for Sestroretsk and Somino samples Δ143Nd/144Nd = 0.000006. The values of Δ87Sr/86Sr are 0.000531, 0.000428 and 0.000016 for pairs of Fe oxyhydroxide–magnetite from Sestroretsk, Polovinkino and Somino, respectively.
Neodymium isotope composition vs. strontium isotope composition for parent iron ore (open symbols) and reduced substance (filled symbols). Asterisk—charcoal, circles—Somino deposit, triangles—bacterial mat with Gallionella spp., Sestra Zavodskaya River(#9b, Table 1), squares—Polovinkino deposit.
DISCUSSION
Data on the natural water and Q-R continental iron ore from the north-western part of EEP and the Baltic craton suggest that their geochemical and isotopic composition is defined by the composition of the beds, which the waters were draining and where iron oxides had been extracted from (Tokarev et al., 2015; Felitsyn and Bogomolov, 2016, 2017; Felitsyn et al, 2019; Svetov and Svetova, 2021). Thus, Nd and Sr isotope composition of Fe oxyhydroxides, studied within the current research, should be following the isotopic composition of aquafacies, where Fe was oxidized and precipitated. Trace element concentration in studied samples, taken together with the results of microbiological research, should provide the basement for estimation the role of Fe-oxidizing bacteria in REE accumulation.
Geochemical Signatures in Recent Bacterial Mats and Iron-Made Artifacts
The enrichment of Fe oxyhydroxides in a number of elements, including REE, is determined by the crystal-chemical properties of goethite group minerals: the adsorption of ions including alkali-earth elements and REE onto Fe oxyhydroxide is a well-known process (e.g. Axe et al., 1998; Trivedi et al., 2001; Tang and Johannesson, 2006; Sajih et al., 2014; Liu et al., 2017; Nie et al., 2017). The surface area of ferrihydrite can reach 840 m2 g–1 (Davis and Leckie, 1978) and oxygen on the ferrihydrite’s surface (Hiemstra and van Riemsdijk, 2009) is considered to be responsible for elements sorption from the water. Thus, the high concentrations of Sm and Nd observed in the group 1 samples (see above) is highly likely to have been determined by the presence of bacterial ferrihydrite.
SEM data which were obtained in the current study, demonstrate that both metallic iron and rust do not contain inclusions of mineral phases, which could distort the REE pattern. Lack of partitioning is supported by almost equal REE patterns (Fig. 3) in the iron parts of artifacts and in their hydrogoethite coating, The REE patterns of rust as observed on the artifacts are the same as the REE patterns in their metallic parts (excluding Ce), so one can assume that there was no addition of REE during oxidation. A smaller amount of Fe (as little as half as much) in the rust cover, compared with the central parts of all the artifacts, and the enrichment of the first ones with REEs (10 times) provides evidence for the withdrawal of iron from the artifacts during the oxidizing in a form of Fe2+. The edging of fine-grained hydrogoethite (1–2 cm width) around the iron-made artifacts found both in soil and timber, would support such a suggestion. The pronounced positive Ce anomaly in rust results from the low mobility of Ce4+ and its accumulation on the Fe hydroxides (Bau 1999; Ohta and Kawabe, 2001). If there is no addition of the REE from soil and wood, the observed Ce enrichment in the rust of the iron-made artifacts points to the removal of a certain part of other light REEs by ground water. At the same time, Ce precipitation in the newly formed rust from the soil was possible.
The addition of REEs from the surrounding wood material is regarded as unlikely, because of a low concentration of REEs in plants (Kabata-Pendias 2010; Felitsyn and Bogomolov, 2016). The enrichment of rust with iron-oxidizing bacteria with REE shows no dependence on the substance surrounding where the artifact was found (wood or soil) as well as the age of the artifacts (Fig. 3). The close values of the Sm/Nd ratios in metallic iron and iron oxyhydroxide (Table 3) also support the assumption of metallic iron being the main source of REEs in rust. The concentration of REEs in the rust coating of the artifacts and in modern day iron items cannot be linked with the Mn hydroxides, because newly formed mineral phases in rust are depleted of Mn.
Bacterial iron oxyhydroxide with Gallionella ferruginea (spring near Sestra Zavodskaya river) shows a bulk REE concentration of 1200 ppm (Felitsyn and Alfimova, 2017). Samples of Fe oxyhydroxide had been carefully purified to remove the accessory minerals—possible REE carriers, and 143Nd/144Nd reported are similar to those found in iron-made artifacts in the same geochemical province in the northwestern part of the East European platform (Felitsyn et al., 2019).
Assuming that Sm and Nd content represents an estimation of bulk REE concentration in rust where remnants of iron bacteria were found, the enrichment factor for REE in Fe oxyhydroxide coating cell walls (up to hundreds of times compared with parent iron alloy, see Table 2) points to the seizure of REE during the oxidization of Fe0, and the formation of iron oxyhydroxide. The comparable enrichment extent discovered for Sr and Rb corroborates the suggestion about the accumulation of elements in bacterial Fe oxyhydroxide from the external source.
The isotopic composition of strontium and neodymium in spring water with biofilms containing iron bacteria on the right bank of Sestra Zavodskaya river (87Sr/86Sr ratios are 0.721388–0.721459, 143Nd/144Nd ratios are 0.511570–0.511592) according (Felitsyn and Alfimova, 2017) is typical for geological provinces with a Proterozoic basement, covered by a Quaternary lacustrine and glacial deposit. The concentrations of Nd and Sr in modern-day iron alloy are rather low, and the adsorption of Nd and Sr from lapping water results in the significant modification of Sr and Nd’s isotopic composition in forming Fe oxyhydroxide cover. The 143Nd/144Nd ratio in the parent metallic material (samples #9 and #10, Fig. 4) exceeds the same in spring water on the bank of the Sestra Zavodskaya river, and the 143Nd/144Nd ratio in the rust for both samples is lower. Metallic substrate from a lath construction contains a lower proportion of radiogenic Sr compared with spring water, and the 87Sr/86Sr ratio value in Fe oxyhydroxide is higher than in the parent iron alloy. Instead, the 87Sr/86Sr ratio value in the metallic substrate in sample #9 (0.765820) has diminished up to 0.747439, influenced by the interaction with the spring water with 87Sr/86Sr = 0.7214.
Thus, a certain distinction had been found in a type of Sr and Nd isotope systematics modification during the oxidization of iron alloys with iron bacteria in rust (samples #9 and #10), and samples of Fe oxyhydroxide without remnants of bacteria (samples ##1–8). In rust with iron-oxydizing bacteria, both isotopic systems are affected, whereas metal—rust pairs with no evidence of iron bacteria display a modification of the Rb-Sr isotope system only (Fig. 4).
Drastic changes in the Sr and Nd concentrations in the process of bacterial corrosion in metal—rust pairs definitely points to intense mass exchange between parent material and newly formed Fe oxyhydroxide. The adsorption of ions including alkali-earth elements and REE onto bacterial ferrihydrite, which is the precursor of observed Fe oxyhydroxides, is a well-known process. Data presented in the current study suggests the enhanced sorption capacity of bacterial Fe minerals compared with rust, without evidence of iron oxidizing bacteria presence. Such a characteristic of bacterial ferrihydrite was described by Chukhrov et al. (1973), based on a laboratory experiment.
The Persistence of Sm–Nd and Rb–Sr Isotope Systems in the Process of Reduction Experiment
The comparison of Rb, Sr, Nd and Sm content in charcoal, iron oxyhydroxide and new minerals points to Fe oxyhydroxide as the main source of the listed elements in Fe2+-bearing minerals and metallic iron mixture, produced in current experiment. At the same time, the isotopic shift in Sr and Nd isotopic composition between parent ore and new iron minerals is rather insignificant and it can be neglected in the practical use of Nd and Sr signatures for geological studies.
During the combustion of charcoal and the reduction of Fe3+ in the closed system of a laboratory furnace, released elements, including trace elements like alkali and alkali-earth elements, incorporate into minerals of iron oxides, goethite and ferrihydrite due to the properties of their crystal lattice (Hiemstra and van Riemsdijk, 2009; Peacock et al., 2016). Iron minerals, including magnetite, are able to accumulate rare earth elements up to tens ppm, which has been examined in iron oxides of different age and genesis (Rhodes et al., 1999; Selmi et al., 2009; Zamanian and Radmard, 2016).The appearance of so-called Cotrell atmosphere (e.g. Reddy et al., 2016) could be responsible for the a accumulation of trace elements (including REEs, Rb, Sr) along the dislocation in Fe minerals’ crystal lattice. The loss of constitutional and/or adsorbed water (up to ~10% wt in studied samples of continental iron ore) does not result in the perceptible modification for the isotopic composition for Nd and Sr in newly formed Fe mineral, as well as the concentration of REE, Sr, Rb compared with parent material.
CONCLUSIONS
Trace element concentrations and Nd and Sm isotopic composition of modern day bacterial ferrihydrite are geochemical proxies of environment it was formed from. A low percentage of iron bacteria within modern day biofilms, which give a significant change in REE and isotopes, restricts the possibility of the micro paleontological and/or biochemical studies of fossilized remnants of such bacteria.
The studied rust cover formed on the ancient (artifacts) and modern iron items demonstrates a significant enrichment of REE and Sr in those samples, which contain active iron-oxidizing bacteria, and for those in which no bacteria was found, enrichment is much lower. The REE patterns in metallic iron and rust cover suggest that metallic iron was the main source of REE in rust cover on the artifacts, and ground waters were the main source of REE for the iron items containing bacteria. Rb–Sr and Sm–Nd isotope systems demonstrate different pathways in biogenic and abiogenic iron oxidation. The rust cover of iron samples with active iron bacteria have a significant shift in 87Sr/86Sr and 143Nd/144Nd compared to the metallic iron, whereas in the absence of the iron bacteria, this shift is observed only for the 87Sr/86Sr ratio.
Iron ores formed from bacterial ferrihydrite under studied experimental conditions (T up to 1000°C) retain the acquired amount of the REE and show no significant shift for isotopic composition of Nd and Sr in pair metallic iron/parent iron ore. Therefore the enhanced concentration of the REE in sedimentary Fe-bearing minerals could be regarded as a marker of its bacterial origin.
Change history
15 November 2023
An Erratum to this paper has been published: https://doi.org/10.1134/S0016702923210036
REFERENCES
N. Alfimova, M. B. Raza, S. Felitsyn, V. Matrenichev, E. Bogomolov, P. Nasipuri, and V. Kumar, “Isotopic Sm-Nd signatures of Precambrian Banded Iron Formation from the Fennoscandian shield, East-European Platform, and Bundelkhand craton, India,” Precambrian Res. 328, 1–8 (2019).
C. R. Andersson and K. Pedersen, “In situ growth of Gallionella biofilms and partitioning of lanthanides and actinides between biological material and ferric oxyhydroxides,” Geobiology 1, 169–178 (2003).
L. Axe, G. B. Bunker, P. R. Anderson, and T. A. Tyson, “An XAFS analysis of strontium at the hydrous ferric oxide surface,” J. Coll. Interf. Sci. 199, 44–52 (1998).
M. Bau, “Scavenging of dissolved yttrium and rare earth by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y–Ho fractionation and lanthanide tetrad effect,” Geochim. Cosmochim. Acta 63, 67–77 (1999)
F. V. Chukhrov, “The problem of genesis of iron pigment in red beds,” In: Hypergene Iron Oxides in Geological Processes, Ed. by N. V. Petrovskaya (Nauka, Moscow, 1975), pp. 126–133 (1975) [in Russian].
F. V. Chukhrov, B. B. Zvyagin, A. I. Gorshkov, L. P. Yermilova, and V. V. Balashova, “On ferrihydrite (hydrous ferric oxide),” Izv. Akad. Nauk SSSR, Geol. Ser. 4, 23–33 (1973).
J. A. Davis and J. O. Leckie, “Surface ionization and complexation at the oxide/water interface II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions,” J. Coll. Interf. Sci. 67, 90–107 (1978).
M. S. Dodd, H. Wang, C. Li, M. Towner, A. R. Thomson, J. F. Slack, and D. Papineau, “Abiotic anoxic iron oxidation, formation of Archean banded iron formations, and the oxidation of early Earth,” Earth Planet. Sci. Lett. 584, 117469 (2022).
D. Emerson, E. J. Fleming, and J. Mcbeth, “Iron-oxidizing bacteria: an environmental and genomic perspective,” Annu. Rev. Microbiol. 64, 561–83 (2010). https://doi.org/10.1146/annurev.micro.112408.134208
S. B. Felitsyn and E. S. Bogomolov, “Rare earth elements and Rb-Sr and Sm-Nd systematics in the peat bog iron ore and moss of the NW East European Platform,” Lithol. Mineral Resour. 51, 107–116 (2016). https://doi.org/10.1134/S0024490216020036
S. B. Felitsyn and N. A. Alfimova, “Isotope and microelement systematics of Gallionella sp.–containing bacterial mats from the northwest of the East European Platform,” Dokl. Biol. Sci. 474, 123–125 (2017). https://doi.org/10.1134/S0012496617030115
S. B. Felitsyn and E. S. Bogomolov, “Neodymium isotope composition in groundwater of St.–Petersburg region,” Geoecology 4, 59–68 (2017).
S. B. Felitsyn, N. A. Alfimova, and E. S. Bogomolov, “Nd and Sr isotopic composition of ancient iron–made artifacts and ores from Northwest Russia,” Geoarchaeology 34, 221–228 (2019). https://doi.org/10.1002/gea.21695
R. Frei, D. Bridgwater, M. Rosing, and O. Stecher, “Controversial Pb-Pb and Sm-Nd isotope results in the early Archean Isua (West Greenland) oxide iron formation: Preservation of primary signatures versus secondary disturbances,” Geochim. Cosmochim. Acta 63, 473–488 (1999).
G. P. Halverson, F. Poitrasson, P. F. Hoffman, C. A. Nédéle, L. J. M. Monte, and J. Kirby, “Fe isotope and trace element geochemistry of the Neoproterozoic syn–glacial Rapitan iron formation,” Earth Planet. Sci. Lett. 309, 100–112 (2011).
R. Haugaard, L. Ootes, R. A. Creaser, and K. O. Konhauser, “The nature of Mesoarchaean seawater and continental weathering in 2.85 Ga banded iron formation, Slave craton, NW Canada,” Geochim. Cosmochim. Acta 194, 34–56 (2016).
T. Hiemstra and W. H. Riemsdijk, “A surface structural model for ferrihydrite I: Sites related to primary charge, molar mass, and mass density,” Geochim. Cosmochim. Acta 73, 4423–4436 (2009).
L. P. Gromet, L. A. Haskin, R. L. Korotev, and R. F. Dymek, The “North American shale composite”: Its compilation, major and trace element characteristics,” Geochim. Cosmochim. Acta 48 (1984). 2469–2482.
A. Kabata-Pendias, Trace Elements in Soils and Plants, 4th edition (CRC Press Taylor & Francis Group, Boca Raton, 2010).
K. O. Konhauser, N. J. Planavsky, D. S. Hardisty, L. J. Robbins, T. J. Warchola, R. Haugaard, and T. W. Lyons, “Iron formations: A global record of Neoarchaean to Palaeoproterozoic environmental history,” Earth–Sci. Rev. 172, 140–177 (2017).
J. Kurz, K. Simon, C. Heim, J. Reitner, N. V. Quéric, and V. Thiel, “Trace element and biomarker signatures in iron-precipitating microbial mats from the Tunnel of Äspö (Sweden),” In: Advances in Stromatolite Geobiology, Ed. by J. Reitner, M. H. Trauth, K. Stüwe, and D. Yuen, (Springer, Berlin–Heidelberg, 2011), pp.221–231.
W. L. Li, B. L. Beard, and C. M. Johnson, “Biologically recycled continental iron is a major component in banded iron formations,” Proc. Nat. Academ. Sci. 112, 8193–8198 (2015). https://doi.org/10.1073/pnas.1505515112
H. Liu, O. Pourret, H. Guo, and J. Bonhoure, “Rare earth elements sorption to iron oxyhydroxide: Model development and application to groundwater,” Appl. Geochem. 87, 158–166 (2017).
Z. Nie, N. Finck, F. Heberling, T. Pruessmann, Ch. Liu, and J. Lützenkirchen, “Adsorption of selenium and strontium on goethite: EXAFS study and surface complexation modeling of the ternary systems,” Environ. Sci. Technol. 51, 3751–3758 (2017).
A. Ohta, and I. Kawabe, “REE (III) adsorption onto Mn dioxide (δ–MnO2) and Fe oxyhydroxide: Ce (III) oxidation by δ–MnO2,” Geochim. Cosmochim. Acta 65, 695–703 (2001).
C. L. Peacock, S. V. Lalonde, and K. O. Konhauser, “Iron minerals as archives of Earth’s redox and biogeochemical evolution,” EMU Notes Mineral. 17, 113–164 (2016).
S. M. Reddy, A. V. Riessen, D. W. Saxey, T. E. Johnson, W. D. A. Rickard, D. Fougerouse, S. Fisher, T. J. Prosa, K. P. Rice, D. A. Reinhard, Y. Chen, and D. Olson, “Mechanisms of deformation–induced trace element migration in zircon resolved by atom probe and correlative microscopy,” Geochim. Cosmochim. Acta 195, 158–170 (2016). https://doi.org/10.1016/j.gca.2016.09.019
A. L. Rhodes, N. Oreskas, and S. Sheets, “Geology and rare earth elements (REE) geochemistry of magnetite deposits at El Laco, Chili,” Soc. Econ. Geol. Spec. Publ. 7, 299–331 (1999).
M. Sajih, N. D. Bryan, F. R. Livens, D. J. Vaughan, M. Descosts, V. Phrommavanh, J. Nos, and R. Morris, “Adsorption of radium and barium on goethite and ferrihydrite: A kinetic and surface complexation modeling study,” Geochim. Cosmochim. Acta, 146, 150–163 (2014).
M. Schad, J. M. Byrne, L. K. Thomas-Arrigo, R. Kretzschmar, K. O. Konhauser, A. Andreas Kappler “Microbial Fe cycling in a simulated Precambrian ocean environment: Implications for secondary mineral (trans)formation and deposition during BIF genesis,” Geochim. Cosmochim. Acta 331, 165–191 (2022).
M. Selmi, L. E. Lagoeiro, and I. Endo, “Geochemistry of hematite and itabirite, Quadrilátero Ferrífero, Brasil,” Rev. Esc. Minas. 62, 35–43 (2009). https://doi.org/10.1590/S037044672009000100006
S. Svetov and E. Svetova, “Trace elements in ferruginous Marcial waters (Russia): chemical stability and mineral assays,” J. Elem. 26, 349–367 (2021). https://doi.org/10.5601/jelem.2021.26.1.2069
J. Tang and K. H. Johannesson, “Controls on the geochemistry of rare earth elements along a groundwater flow path in the Carrizo Sand aquifer, Texas, USA,” Chem. Geol. 225, 156–171 (2006).
I. V. Tokarev, I. V. Blazhennikova, I. A. Avramenko, and G. S. Borodulina, “Isotope–geochemical data on ferruginous mineral waters: conditions of formations of “marcial waters” resort, Karelia,” Geochem. Int. 53, 83–86 (2015).
P. Trivedi, L. Axe, and J. Dyer, “Adsorption of metal ions onto goethite: single–adsorbate and competitive systems,” Colloid. Surf. A 191, 107–121 (2001).
S. Viehmann, M. Bau, J. E. Hoffmann, and C. Muenker, “Geochemistry of the Krivoy Rog Banded Iron Formation, Ukraine, and the impact of peak episodes of increased global magmatic activity on the trace element composition of Precambrian seawater,” Precambrian Res. 270, 165–180 (2015). https://doi.org/10.1016/j.precamres.2015/09.015
H. Zamanian and K. Radmard, “Geochemistry of rare earth elements in the Baba Ali magnetite skarn deposit, western Iran – a key to determinate conditions of mineralization,” Geologos 22, 33–47 (2016). https://doi.org/10.1515/logos-2016-003
ACKNOWLEDGMENTS
The authors are thankful to Mr. J.B. Moyes for his help with the English language usage and to reviewers Drs. A.D. Slukin and N.M. Boeva. The work was performed within the framework of government contract FMUW-2022-0004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Felitsyn, S., Alfimova, N. & Bogomolov, E. The Accumulation of the REE by Bacterial Fe Oxyhydroxide. Geochem. Int. 61, 1442–1455 (2023). https://doi.org/10.1134/S0016702923090021
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702923090021