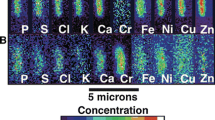
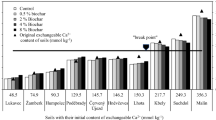
Abstract
The paper addresses complexation of metal ions with humic matter in soils. The functional specifics of humic matter extracted from gley-podzolic soils are estimated using spectrometric techniques. Conditional stability constants are experimentally determined for Fe(III), Cu(II), Pb(II), Cd(II), Zn(II), Ni(II), Co(II), Mn(II), Cr(III), Ca(II), Mg(II), Sr(II), and Al(III). The activities of metals are ranked according to their affinity to humic compounds in soils. The determined conditional stability constants of the complexes are tested in model experiments, and it is demonstrated that Fe and Al ions have higher conditional stability constants than ions of alkali-earth metals, Pb, Cu, and Zn.
Similar content being viewed by others
References
H. E. Allen and C. Boonayangoor, “Changes in physicochemical forms of lead and cadmium added to fresh water,” Environ. Int. 7(5), 337–341 (1982).
P. Bassini and U. Sutter, “An experimental heavy metal pollution study. Chemical speciation and biological availability of copper in lake water,” Hydrol. (Schweiz. Z.). 41(3), 291–314 (1979).
J. N. Butler, Ionic Equilibrium: A Mathematical Approach (Addison-Wesley, Reading, MA, 1964).
M. E. Bender, W. R. Matson, and R. A. Jordan, “On the significance of metal complexing agents in secondary sewage effluents,” Environ. Sci. Technol. 4(6), 520–521 (1970).
P. Benes, E. Gjessing, and E. Steinnes, “Interactions between humus and trace elements in fresh water,” Water Res. 10(8), 711–716 (1976).
P. Benes and E. Steinnes, “Migration forms of trace elements in natural fresh waters and the effect of the water storage,” Water Res. 9(8), 741–749 (1975).
J. Buffle and F. L. Greter, “Voltammetric study of humic and fulvic substances. Part II. Mechanism of reaction of the fulvic complexes on the mercury electrode,” J. Electroanal. Chem. 101(2), 231–251 (1979).
J. Buffle, F. L. Greter, and W. Haerdi, “Measurement of complexation properties of humic and fulvic acids in natural waters with lead and copper ion-selective electrodes,” Anal. Chem. 49(2), 216–222 (1977).
Ò. Koljonen and L. Carlson, “Behavior of the major elements and minerals in sediments of four humic lakes in southeastern Finland,” Soc. Geograph. Fenn. 5, 47–89 (1975).
N. N. Danchenko, Functional Composition of Humic Acids: Determination and Interrelation with Reaction Ability (MGU, Moscow, 1997) [in Russian].
C. Feller and M. Brossard, “Selected pioneering works on humus in soils and sediments during the 20th century: A retrospective look from the International Humic Substances Society view,” Phys. Chem. Earth. 35, 903–912 (2010).
J. M. Garcia-Mina, M. C. Antolín, and M. Sánchez-Diaz, “Metal-humic complexes and plant micronutrient uptake: a study based on different plant species cultivated in diverse soil types,” Plant Soil 258, 57–68 (2004).
I. G. Gorichev and A. D. Izotov, et al., An Application of Concepts on the Structure of Double Electric Layer in Methods of Experimental Determination and Calculation of Constants of Acid-Basic Equilibria at the Oxide/Electrolyte Boundary (RUDN, Moscow, 2001) [in Russian].
Ya. Intsedi, Application of Complexes in Analytical Chemistry (Mir, Budapest, 1979) [in Russian].
S. Karavoltsos and A. Sakellari, “Copper complexation in wet precipitation: impact of different ligand sources,” Atmosph. Environ. 80, 13–19 (2013).
D. V. Kovalevsky, Extended Abstract of Candidate’s Dissertation in Chemistry, (MGU, Moscow, 1998) [in Russian].
D. V. Kovalevsky, A. B. Permin, I. V. Perminova, and V. S. Petrosyan, “Choice of registration conditions of quantitative C(13) NMR spectra of humic acids,” Vestn. Mosk. Gos. Univ. Ser. 2. Chem., 41(1), 39–41 (2000).
D. Kreller and M. Schlautman, “Combined HPLC/HPSEC study of Suwanee River fulvic acid adsorptive fractionation on α-aluminum oxide,” J. Colloid Interface Sci. 390, 242–249 (2013).
J. Leenheer and J. Croue, “Characterization aquatic dissolved organic matter,” Environ. Sci. Technol. 37, 18–26 (1999).
I. A. Linnik and B. I. Nabivanets, Speciation of Metal Migration in Fresh Surface Waters (Gidrometizdat, Leningrad, 1986) [in Russian].
P. N. Linnik, “Humic matters and their significance of aqueous systems,” Gidrobiol. Zh. 40(91), 81–107 (2009).
J. N. Murrell, S. F. A. Kettle, and J. M. Tedder, The Chemical Bond (Wiley, New York, 1978).
T. M. Minkina, G. V. Motuzova, O. G. Nazarenko, V. S. Kryshchenko, and S. S. Mandzhieva, “Forms of heavy metal compounds in soils of the steppe zone,” Euras. Soil Sci. 41(7), 708–716 (2008).
T. I. Moiseenko, I. V. Rodyushkin, V. A. Duval’ter, and L. P. Kudryavtseva, Formation of Quality of Surface Waters and Bottom Deposits under Anthropogenic Loads on the Water Drainage Areas of the Arctic Basin with Reference to the Kola North (Kol’sk. nauch. tsentr, Apatity, 1996) [in Russian].
T. I. Moiseenko, L. P. Kudryavtseva, and N. A. Gashkina, Trace Elements in the Onland Surface Waters: Technophile Properties, Bioaccumualtion, and Ecolotoxicology (Nauka, Moscow, 2006) [in Russian].
D. S. Orlov, O. N. Biryukova, and N. I. Sukhanova, Organic Matter in Soils of the Russian Federation (Nauka, Moscow, 1996) [in Russian].
D. M. Ozdoba, J. C. Blyth, R. F. Engler, H. Dinel, and M. Schnitzer, “Leonardite and humified organic matter,” in Proc. Humic Substances Seminar (Boston, 2001).
F. D. Paolid and J. Kukkonen, “Binding of organic pollutants to humic and fulvic acids: influence of pH and the structure of humic material,” Chemosphere 34(8), 1693–704 (1997).
A. I. Perel’man, Geochemistry (Vyssh. shkola, Moscow, 1989) [in Russian].
I. V. Perminova and N. N. Danchenko, “Detoxication of heavy metals, polyaromatic carbohydrate, and pesticides by humic matters in waters and soils,” in Proceedings of International Water Congress: Ecology and Technology, Moscow, Russia (Atlant, Moscow,), pp. 1136–1143 [in Russian].
M. A. Ryazanov, V. A. Beznosikov, and E. D. Lodygin, “Evaluation of the acid-base properties of fulvic acids using pK spectroscopy,” Euras. Soil. Sci. 34(8), 830–836 (2001).
S. Sachs, M. Bubner, K. Schmeide, G. R. Choppin, K. H. Heise, and G. Bernhard, Carbon-13 NMR Spectroscopic Studies on Chemically Modified and Unmodified Synthetic and Natural Humic Acids (Talanta, New York, 2002).
M. Schnitzer and S. U. Khan, Human Substances (New York, 1972).
G. Schuman, “Molecular modeling of soil organic matter: Squaring the circle?” Geoderma, 166, 1–14 (2011).
G. L. Shlefer, Complexation in Solutions (Khimiya, Moscow, 1969) [in Russian].
B. S. Smolyakov and V. I. Belevantsev, “Chemical species of copper, cadmium, lead in fresh-water basins,” Khim. Int. Ustoich. Razvitiya 7, 575–583 (1999).
M. Vandenbroucke, “From types to models of chemical structure,” Oil Gas Sci. Technol. 58(2), 243–269 (2003).
G. M. Varshal, Doctoral Dissertation in Chemistry (Inst. Geokhim. Analit. Khim. RAN, Moscow, 1994) [in Russian].
F. Vydra, K. Shtulik, and E. Yulakova, Invasion Voltammetry (Mir, Moscow, 1980) [in Russian].
A. G. Zavarzina, N. G. Vanifatova, and A. A. Stepanov, “Fractionation of humic acids according to their hydrophobicity, size, and charge-dependent mobility by the salting-out method,” Euras. Soil. Sci. 41(12), 1294–1301 (2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.I. Dinu, 2015, published in Geokhimiya, 2015, No. 3, pp. 276–288.
Rights and permissions
About this article
Cite this article
Dinu, M.I. Interaction between metal ions in waters with humic acids in gley-podzolic soils. Geochem. Int. 53, 265–276 (2015). https://doi.org/10.1134/S0016702915030052
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0016702915030052