Abstract
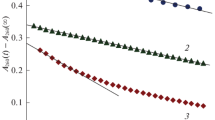
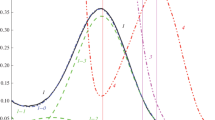
The solubility of ozone (O3) in 5 M solutions of H2SO4 or НСlO4 and in 18 M H2SO4 markedly increases as the temperature decreases from 25 to–70°C. In particular, the solubility coefficient of ozone (α = [Ο3]liq/[Ο3]gas) in 5 M H2SO4 increases in this temperature range from 0.13 to 1.47, the heat of solubility being 13.5 kJ/mol. At low temperature, the absorption band of ozone at 588 nm (ε = 4.5 L mol–1 cm–1) is detected. At T ≤–30°C, ozone virtually does not decompose in acid solutions.
Similar content being viewed by others
References
Yoneda, N. and Olah, G.A., J. Am. Chem. Soc., 1977, vol. 99, no. 9, pp. 3113–3119.
Kolenchin, N.F., Usp. Sovrem. Estestvozn., 2011, no. 3, pp. 78–79.
Krylova, L.N., Panin, V.V., Samoilovich, V.G., and Voronin, D.Yu., VI nauchno-prakticheskaya konferentsiya “Moskovskaya nauka—problemy i perspektivy” (VI Scientific and Practical Conference “Moscow Science—Problems and Prospects”) 2005, Moscow, 2005, pp. 416–425.
Ershov, B.G., Morozov, P.A., Gordeev, A.V., and Seliverstov, A.F., Khim. Tekhnol. Vody, 2009, vol. 31, no. 6, pp. 665–676.
Tyupalo, N.F., Lutsyk, A.I., Semenyuk, T.N., and Dmitruk, A.F., Dokl. Akad. Nauk SSSR, 1987, vol. 297, no. 3, pp. 624–627.
Levanov, A.V., Kuskov, I.V., Antipenko, E.E., and Lunin, V.V., Zh. Fiz. Khim., 2008, vol. 82, no. 7, pp. 1275–1281.
Speranza, M., J. Phys. Chem. A, 1998, vol. 102, no. 38, pp. 7535–7536.
Bader, H. and Holgne, J., Ozone: Sci. Eng., 1982, vol. 4, no. 4, pp. 169–176.
Panich, N.M. and Ershov, B.G., Zh. Fiz. Khim., 2008, vol. 82, no. 8, pp. 1423–1426.
Steinfeld, J.I., Adler-Golden, S.M., and Gallagher, J.W., J. Phys. Ref. Data, 1987, vol. 16, no. 4, pp. 911–951.
Taube, H., Trans. Faraday Soc., 1957, vol. 53, pp. 656–665.
Hart, E.J., Sehested, K., and Holcman, J., Anal. Chem., 1983, vol. 55, no. 1, pp. 46–49.
Cacace, F. and Speranza, M., Science, 1994, vol. 265, no. 5169, pp. 208–209.
Zakharov, I.I., Kolbasina, O.I., Semenyuk, T.N., Tyupalo, N.F., and Zhidomirov, G.M., Zh. Strukt. Khim., 1993, vol. 34, no. 2, pp. 28–32.
Aleksandrov, Yu.A., Tarunin, B.I., and Perepletchikov, M.L., Zh. Fiz. Khim., 1983, vol. 57, no. 10, pp. 2385–2397.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © B.G. Ershov, N.M. Panich, 2015, published in Doklady Akademii Nauk, 2015, Vol. 465, No. 2, pp. 190–193.
Rights and permissions
About this article
Cite this article
Ershov, B.G., Panich, N.M. The solubility and decomposition of ozone in solutions of sulfuric and perchloric acids in the temperature range from 25 to –70°C. Dokl Phys Chem 465, 279–282 (2015). https://doi.org/10.1134/S0012501615110068
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012501615110068