Abstract
The article focuses on the pathogenetic mechanisms of posttraumatic stress disorder (PTSD), which is associated with psychological stress because of the coronavirus pandemic. The molecular mechanisms responsible for disease susceptibility in some individuals and stress resistance in others are amongst crucial research interests of experimental and clinical medicine. Priority data were obtained to indicate that distortions of synthesis and metabolism and, most significantly, a switch between two energy transport forms, glucose and lipids, underlie myocardial dysfunction in young and old stress-sensitive Wistar rats in a PTSD model. Histochemistry and polarization microscopy showed energy deficit in cardiomyocytes and signs of ischemic and hypoxic areas emerging in the myocardium as a result of an accumulation of NADH and NADPH, which initiate excessive production of reactive oxygen species.
Similar content being viewed by others
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a severe mental condition and results from single or repeated incidents that exert a dramatic negative effect on the patient’s mental state. PTSD manifestation is delayed in the majority of cases and is characterized by mental disorders accompanied by somatic pathologies. PTSD does not uniformly develops in all individuals because of many factors, including hereditary susceptibility to stress [1]. The set of PTSD-associated diseases includes cardiovascular and other disorders, which are underlain not only by neuroendocrine alterations, but also by distorted metabolism of amino acids and lipids, insulin resistance, etc. [2]. However, features of substance and energy metabolism have not been compared as of yet between individuals differing in stress resistance or age.
The objective of this work was to study the morphological and functional state of the myocardium and the features of the lipid profile and energy transport forms in stress-sensitive and stress-resistant or young and all individuals, using a PTSD model in Wistar rats.
MATERIAL AND METHODS
We used adult male Wistar rats aged 5.5–6.0 (n = 60, body weight 210–230 g) and 21.6–23.8 (n = 60, body weight 670–850 g) months. The rats were kept in standard housing conditions with free access to water and standard complete granulated fodder.
A common predator stress model, which is based on rodent fear of predators and their smell, was used to reproduce PTSD. Rats were exposed to cat urine for 10 days at 10 min/day and then kept in standard housing conditions for 14 days. Behavioral and biochemical parameters were assessed before modeling PTSD and at the end of the experiment; morphological and morphometric parameters of the myocardium were determined. All experimental procedures were carried out in compliance with Directive 2010/63/EU of the European Parliament of September 22, 2010 on the protection of animals used for scientific purposes.
To study the behavioral manifestations of PTSD, rats were tested in an elevated plus maze (EPM, 600 s). The anxiety index (AI) was calculated according to Cohen et al. [3]: AI = 1 – [(TOA/TD + EOA/TE)/2], where TOA is the total time spent in open arms, TD is the test duration (600 s), EOA is the number of entries to the open arms, and TE is the total number of entries to the EPM arms.
Blood samples for biochemical testing were collected in the morning on an empty stomach. The serum corticosterone concentration was measured by ELISA (IBL, Germany). Glucose, total cholesterol (TCh), high density lipoprotein cholesterol (HDL-Ch), low density lipoprotein cholesterol (LDL-Ch), and triglycerides (TG) were measured using a CardioChek PA blood chemistry analyzer (United States). The very low density lipoprotein cholesterol (VLDL-Ch) content was calculated as VLDL-Ch = TG/2.2 [4]; the atherogenicity coefficient (AC), as AC = (TCh – HDL-Ch)/HDL-Ch; and Castelli index (CI), as CI = TCh/HDL-Ch [5, 6].
In histomorphology analysis, myocardial samples were collected from the left ventricle, fixed with neutral 10% formaline, dehydrated, embedded in Histomix, and used to obtain 5-µm sections. Unstained histological sections were examined by polarized light microscopy; glycogen was detected using periodic acid Schiff stain. Ten images of unstained myocardial sections and ten images of stained ones were obtained for each rat with an Axioplan 2 imaging microscope equipped with a digital camera and an image processing system (Carl Zeiss MicroImaging, Germany). The optical density of a section was determined using ImageJ software (Fiji). All images were obtained in the same conditions; relative values were used; measurements were performed in pixels.
Test groups were compared using ANOVA, the Kruskal–Wallis test for multiple comparisons, and the Mann–Whitney U-test for pairwise comparisons. Results were presented as median (lower quartile; upper quartile) (Me (Q1; Qu)). Differences were considered significant at p ≤ 0.05.
RESULTS AND DISCUSSION
Testing with an EPM showed that the AI, which provides a main integral behavioral characteristic, in stress-sensitive rats was significantly higher than in stress-resistant rats before PTSD modeling (p < 0.01, Fig. 1). The test was repeated after stress exposure and showed that the AI of stress-sensitive rats increased in both age groups by 23.1% (p < 0.01) and that the AI of stress-resistant rats did not differ from its control value (p > 0.05, Fig. 1). Based on the discriminant analysis of AI values observed in the intact and stressed rats, rats with AI > 0.80 were considered stress sensitive and those with AI < 0.80, stress resistant, like in our previous experiments [7].
Behavioral parameters in stress-sensitive and stress-resistant Wistar rats of different ages upon PTSD modeling. SSYI, stress-sensitive young intact rats; SRYI, stress-resistant young intact rats; SSYPTSD, stress-sensitive young rats with PTSD; SRYPTSD, stress-resistant young rats with PTSD; SSOI, stress-sensitive old intact rats; SROI, stress-resistant old intact rats; SSOPTSD, stress-sensitive old rats with PTSD; SROPTSD, stress-resistant old rats with PTSD. EOA, number of entries to the open arms (with a coefficient of 101); TE, total number of entries to the arms (with a coefficient of 101); TOA, total time spent in the open arms; AI, anxiety index (with a coefficient of 102). Differences in AI (*) from the control group and (#) between the stress-resistant and stress-sensitive subgroups were significant at p < 0.01.
Corticosterone measurements provided additional evidence that a PTSD-like condition developed in rats exposed to predator stress. The corticosterone level decreased in both of the age groups (p < 0.01, Table 1). It should be noted that the corticosterone level already differed between the stress-resistant and stress-sensitive rat subgroups before stress exposure. Both young and old stress-sensitive rats had higher corticosterone levels as compared with the respective stress-resistant rats (Table 1).
Rat blood lipid profiling (Table 1) showed that proatherogenic (in humans) factors in the intact stress-sensitive subgroups were higher than in the stress-resistant subgroups (p < 0.01). The difference became greater after PTSD modeling (p < 0.01). Note that changes in lipid profile in rats are not as great as in humans and that signs of atherosclerosis do not develop in rats. A greater range of parameters was therefore assessed in this work than in studies of other researchers in order to obtain more informative data and to allow the findings to be extrapolated to humans.
When the AC and CI were compared, the latter was found to be more sensitive to the experimental conditions. Higher CI values were observed in all of the old rat subgroups and in the stress-sensitive young group (p < 0.001), while higher AC values were detected only in old rats exposed to stress (p < 0.001). A glucose to triglyceride ratio proved the most informative, allowing us to analyze the strength and extent of the effects exerted by the factors that induce a change from glucose as a normally main energy transport form to lipids. Predominant mobilization of free fatty acids is usually observed at an old age and on exposure to extreme factors [8]. A dramatic significant change was observed in both age groups upon PTSD modeling and in intact old rats of the two subgroups in our experiment. The liver is a main organ involved in producing and metabolizing glucose and lipids as two energy sources. Changes in the blood concentrations of the two energy transport forms reflect distorted metabolism of glucose and lipids in the liver according to our previous findings [9]. Combined with morphological and functional alterations of the liver, the changes detected in lipid profile in our experiment may act as important factors in the development of cardiovascular disorders even without inducing atherosclerotic lesions, especially in stress-sensitive rats.
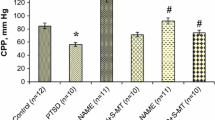
The morphological and functional state of the myocardium was consequently studied in stress-resistant vs. stress-sensitive and young vs. old rats. In the normal conditions, the optical densities of sections examined by polarized light microscopy or stained for glycogen with periodic acid Schiff stain did not significantly differ between stress-resistant and stress-sensitive rats in both of the age subgroups (p > 0.05). A difference was detected only between old and young rats; i.e., glycogen contents of all old rats were considerably lower than in the young rats (p < 0.001, Fig. 2). In the PTSD model, a distinct decrease in glycogen content was observed in young stress-sensitive rats compared with the control (p < 0.001), while the glycogen level remained within its normal range in stress-resistant rats (p > 0.05). Glycogen contents in the two old rat subgroups exposed to stress were lower than normal for the age (p < 0.001), being especially low in stress-sensitive rats (p < 0.001, Fig. 2).
Optical densities of myocardial sections stained for glycogen or examined by polarized light microscopy in stress-resistant and stress-sensitive Wistar rats of different ages in normal conditions and a PTSD model. Rat groups are designated as in Fig. 1. PAS, periodic acid Schiff staining for glycogen; PLM, polarized light microscopy. Differences (*) from the respective initial value, (#) between the stress-resistant and stress-sensitive subgroups, and (^) between the young and old rat subgroups were significant at p < 0.05.
Polarized light microscopy, which detects cardiomyocyte autofluorescence, showed that PTSD modeling decreased the optical density in the two young rat subgroups compared with intact rats and only in the stress-sensitive subgroup of the old rat group (p < 0.001, Fig. 2). Especially low values were observed in the stress-sensitive rats, especially old ones (p < 0.001, Fig. 2). A decrease in optical density characterizing cardiomyocyte autofluorescence is a well-known phenomenon that is used to assess the degree of myocardial ischemia and/or hypoxia. Pyridine and flavin nucleotides found in the cytosol and mitochondria of cardiomyocytes are a main source of their autofluorescence [10]. An accumulation of nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH) determines production of reactive oxygen species and thus triggers lipid peroxidation in cardiomyocytes [11]. Thus, ischemia and, in some cases, hypoxia as a distortion of tissue and cell respiration developed in the myocardium upon PTSD modeling in the two young rat subgroups and the stress-sensitive old rat subgroup in our experiment. It is possible to conclude by extrapolating the findings to humans that coronary heart disease developing as a result of stress at a young age may progress and cause heart failure and other cardiovascular disorders to reduce the working capacity and to cause disability. Myocardial ischemia is even more dangerous at an old age.
The relationship between disease and behavior has always been of interest in philosophy, medicine, and research. A tight association between coronary heart disease and mental disorders has been demonstrated to date. The association works both ways; i.e., there is evidence that one disease can provoke the other. Patients with severe mental disorders, including schizophrenia, depressive conditions, anxiety disorders, and PTSD, are at higher risk of coronary heart disease as compared with the control population according to prospective epidemiological data. On the other hand, mental signs of the above disorders are often found in patients with coronary heart disease [12]. Common etiological mechanisms certainly underlie mental and cardiovascular disorders, the set including biological, behavioral, psychological, and genetic mechanisms. At the same time, a single risk factor may increase the risk for several other diseases.
Stress-sensitive rats displayed overt signs of anxiety and higher blood concentrations of non-esterified fatty acids as compared with stress-resistant rats of the two age groups in our experiment. Although atherosclerosis does not develop in laboratory rodents, autofluorescence zones suggestive of ischemia and/or hypoxia were found in myocardial samples from rats of these groups. Higher anxiety combined with an increase in blood non-esterified fatty acids may therefore serve as a predictor of heart disorders. An increasing body of data accumulated in recent years associates higher concentrations of the fatty acids in question with cardiovascular disorders and their risk factors [13, 14]. The source of higher non-esterified fatty acid concentrations is still unclear. However, it cannot be excluded that pharmacological agents that modulate the central system governing the stress response may have a beneficial effect in treating cardiovascular disorders. To summarize, we were the first to directly detect a dramatic decrease in glycogen as a main energy depot in cardiomyocytes and to observe signs of ischemic and hypoxic zones arising in the myocardium in a Wistar rat model of PTSD. The mechanisms that induce these processes were established to include distortions in metabolism, synthesis, transport, and predominance of blood glucose and lipids as main energy forms. Based on polarized light microscopy findings, NADH and NADPH accumulate in cardiomyocytes and change their redox state to trigger excess production of reactive oxygen species and, accordingly, lipid peroxidation. Our priority finding is that the above pathogenetic mechanisms are most intense in young and old stress-sensitive rats. The finding that the CI and glucose-to-triglyceride ratio are highly sensitive to the experimental conditions can be used to study the strength and extent of the effects exerted by the factors under study. Once additional studies are performed, the glycogen level in cardiomyocytes may provide a prognostic trait to be assessed to plan prevention and treatment.
REFERENCES
Osório, C., Probert, T., Jones, E., et al., Adapting to stress: understanding the neurobiology of resilience, Behav. Med., 2017, vol. 43, no. 4, pp. 307–322.
Somvanshi, P.R., Mellon, S.H., Flory, J.D., et al., Mechanistic inferences on metabolic dysfunction in posttraumatic stress disorder from an integrated model and multiomic analysis: role of glucocorticoid receptor sensitivity, Am. J. Physiol. Endocrinol. Metab., 2019, vol. 317, no. 5, pp. E879–E898.
Cohen, H., Tianmin, L., Kozlovsky, N., et al., The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder, Neuropsychopharmacology, 2011, vol. 37, no. 2, pp. 350–363.
Friedewald, W.T., Levy, R.I., and Fredrickson, D.S., Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge, Clin. Chem., 1972, vol. 18, no. 6, pp. 499–502.
Klimov, A.N., Preventivnaya kardiologiya (Preventive cardiology), Moscow: Meditsina, 1977.
Castelli, W.P., Abbott, R.D., and McNamara, P.M., Summary estimates of cholesterol used to predict coronary heart disease, Circulation, 1983, vol. 67, no. 4, pp. 730–734.
Manukhina, E.B., Tseilikman, V.E., Komelkova, M.V., et al., Cardiac injury in rats with experimental posttraumatic stress disorder and mechanisms of its limitation in experimental posttraumatic stress disorder-resistant rats, J. Appl. Physiol., 2021, vol. 130, no. 3, pp. 759–771.
Won, B.Y., Park, S.G., Lee, S.-H., et al., Characteristics of metabolic factors related to arterial stiffness in young and old adults, Clin. Exp. Hypertens., 2020, vol. 42, no. 3, pp. 225–232.
Kondashevskaya, M.V., Experimental evaluation of the effects of low-dose heparin on the behavior and morphofunctional status of the liver in Wistar rats with posttraumatic stress disorders, Bull. Exp. Biol. Med., 2018, vol. 164, no. 4, pp. 488–492.
Chance, B., Cohen, P., Jobsis, F., et al., Intracellular oxidation-reduction states in vivo, Science, 1962, vol. 137, no. 3529, pp. 499–508.
Papayan, G., Petrishchev, N., and Galagudza, M., Autofluorescence spectroscopy for NADH and flavoproteins redox state monitoring in the isolated rat heart subjected to ischemia-reperfusion, Photodiagn. Photodyn. Ther., 2014, vol. 11, no. 3, pp. 400–408.
De Hert, M., Detraux, J., and Vancampfort, D., The intriguing relationship between coronary heart disease and mental disorders, Dialogues Clin. Neurosci., 2018, vol. 20, no. 1, pp. 31–40.
Gharipour, M., Sadeghi, M., Nezafati, P., et al. Cardiovascular disease risk assessment: triglyceride/high-density lipoprotein versus metabolic syndrome criteria, J. Res. Health Sci., 2019, vol. 19, no. 2, art. ID e00442.
Ivanova, A.Yu., Rysenkova, E.Yu., Afanas’ev, M.A., Chumachenko, P.V., Popov, V.S., Postnov, A.Yu., Medvedeva, N.A., and Medvedev, O.S., Changes in the morphological and functional parameters of the cardiovascular system against the background of a diet with high calorie content in spontaneously hypertensive rats, Klin. Eksp. Morfol., 2021, vol. 10, no. 1, pp. 50–57.
Funding
This work was performed as part of the basic portion of a state assignment to the Avtsyn Institute of Human Morphology and supported by the Foundation for Promising Research of the Chelyabinsk State University and by Institute of Immunology and Physiology, Ural Branch, Russian Academy of Sciences, Yekaterinburg, Russia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All experimental procedures were carried out in compliance with Directive 2010/63/EU of the European Parliament of September 22, 2010 on the protection of animals used for scientific purposes.
Additional information
Translated by T. Tkacheva
Rights and permissions
About this article
Cite this article
Kondashevskaya, M.V., Tseilikman, V.E., Komelkova, M.V. et al. Risk Factors and Mechanisms of Cardiovascular Diseases in Posttraumatic Stress Disorder Model in Wistar Rats as Dependent on Stress Resistance and Age. Dokl Biol Sci 505, 95–99 (2022). https://doi.org/10.1134/S0012496622040020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012496622040020