Abstract
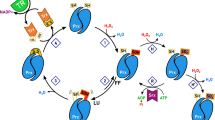
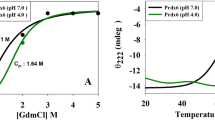
The comparative characterization of thermal stability of human peroxiredoxins 1–6 (Prx1–Prx6) has been performed by physicochemical and biochemical methods and the role of disulfide bonds in stabilizing their structure has been shown. Prx1 and Prx2 among the tested peroxiredoxins exhibit the highest peroxidase activity and thermal stability. Prx1 and Prx2 are more than 2 times more active on average with H2O2 and tert-butyl hydroperoxide as substrates compared to other peroxiredoxins and retain at least 50% activity after 30 min heating at a temperature of 64°C, which is more than 10°C higher compared to Prx3–Prx6. The reduction of the disulfide bonds between Prx1 and Prx2 leads to a decrease of their thermal stability, comparable to the thermal stability of Prx3–Prx6, which confirms the important role of the intermolecular S–S bonds in stabilizing the structure of these proteins.
Similar content being viewed by others
Abbreviations
- Prx:
-
peroxiredoxin(s)
References
A. Perkins, L. B. Poole, and P. A. Karplus, Biochemistry 53, 7693 (2014).
S. G. Rhee, Mol. Cells 39, 1 (2016).
E. M. Hanschmann, J. R. Godoy, C. Berndt, et al., Antioxid. Redox Signal. 19, 1539 (2013).
Z. A. Wood, E. Schröder, J. R. Harris, and L. B. Poole, Trends Biochem. Sci. 28, 32 (2003).
J. L. Pan and J. C. Bardwell, Protein Sci. 15, 2217 (2006).
A. Perkins, K. J. Nelson, J. R. Williams, et al., Biochemistry 52, 8708 (2013).
S. G. Rhee and I. S. Kil, Annu. Rev. Biochem. 85, 1 (2016).
J. König, H. Galliardt, P. Jütte, et al., J. Exp. Bot. 64, 3483 (2013).
F. Angelucci, A. Bellelli, M. Ardini, et al., FEBS J. 282, 2827 (2015).
M. G. Sharapov, V. I. Novoselov, and V. K. Ravin, Mol. Biol. (Moscow) 43 (3), 505 (2009).
S. W. Kang, I. C. Baines, and S. G. Rhee, J. Biol. Chem. 273, 6303 (1998).
M. G. Sharapov, V. I. Novoselov, E. E. Fesenko, et al., Free Radic. Res. 51 (2), 148 (2017).
P. L. Privalov, E. I. Tiktopulo, and N. N. Khechinashvili, Int. J. Pept. Protein Res. 5 (4), 229 (1973).
N. F. Bunkin, A. V. Shkirin, N. V. Suyazov, et al., J. Phys. Chem. B 120, 1291 (2016).
W. Lee, K. S. Choi, J. Riddell, et al., J. Biol. Chem. 282 (30), 22011 (2007).
F. Angelucci, F. Saccoccia, M. Ardini, et al., J. Mol. Biol. 425, 4556 (2013).
M. G. Sharapov, V. K. Ravin, and V. I. Novoselov, Mol. Biol. (Moscow) 48 (4), 600 (2014).
T. J. Tavender, J. J. Springate, and N. J. Bulleid, EMBO J. 29, 4185 (2010).
R. M. Jarvis, S. M. Hughes, and E. C. Ledgerwood, Free Radic. Biol. Med. 53, 1522 (2012).
H. Nassour, Z. Wang, A. Saad, et al., Sci. Rep. 6, 29389 (2016).
M. C. Sobotta, W. Liou, S. Stocker, et al., Nat. Chem. Biol. 11, 64 (2015).
M. Fernandez-Caggiano, E. Schroder, H. J. Cho, et al., J. Biol. Chem. 291, 10399 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.G. Sharapov, N.V. Penkov, S.V. Gudkov, R.G. Goncharov, V.I. Novoselov, E.E. Fesenko, 2018, published in Biofizika, 2018, Vol. 63, No. 2, pp. 232–240.
Rights and permissions
About this article
Cite this article
Sharapov, M.G., Penkov, N.V., Gudkov, S.V. et al. The Role of Intermolecular Disulfide Bonds in Stabilizing the Structure of Peroxiredoxins. BIOPHYSICS 63, 154–161 (2018). https://doi.org/10.1134/S0006350918020203
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350918020203