Abstract
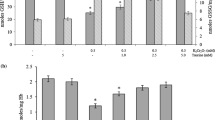
In vitro exposure of human erythrocytes to H2O2 at concentrations of 30–1000 μM resulted in a dose-dependent increase of the intracellular levels of Zn2+ and inhibition of the cytosolic esterase activity, which is a major marker of erythrocyte viability. The observed effect depended on the concentration of H2O2 and the duration of exposure of the cells to this compound. An inverse relationship between the changes in the intracellular level of labile zinc ions and esterase activity in the cells exposed to hydrogen peroxide was detected; this was indicative of the role of Zn2+ in the programmed death of red blood cells. The combined action of hydrogen peroxide and N',N'-tetrakis-(2-pyridyl-methyl)-ethylenediamine, an intracellular zinc ion chelator, has been found to eliminate the cytotoxic effect of H2O2, whereas the addition of Zn2+ to the erythrocyte incubation medium enhanced the effects of hydrogen peroxide. The reduction of the concentration of non-protein thiol groups due to a decrease of the level of reduced glutathione was shown to contribute to the release of Zn2+ from the intracellular binding sites during oxidative stress induced by H2O2 in human erythrocytes.
Similar content being viewed by others
Abbreviations
- TPEN, N’:
-
N’-tetrakis-(2-pyridyl-methyl)-ethylenediamine
- NEM:
-
N-ethylmaleimide
- NAC:
-
N-acetyl-L-cysteine
- GSH:
-
reduced glutathione
References
Yu. M. Harmaza and E. I. Slobozhanina, Biophysics (Moscow) 59 (2), 264 (2014).
J. M. Berg, Science 271 (5252), 1081 (1996).
E. Okazaki, L. Chikahisa, K. Kanemaru, and Y. Oyama, Jpn. J. Pharmacol. 71 (4), 273 (1996).
Y. Sakanashi, T. M. Oyama, Y. Matsuo, et al., Toxicol. In Vitro 23 (2), 338 (2009).
Yu. M. Harmaza, A. V. Tamashevskii, N. V. Goncharova, and E. I. Slobozhanina, Novosti Mev.-Biol. Nauk 3 (1), 90 (2011).
Y. Harmaza and E. Slobozhanina, FEBS J. 280 (1), 218 (2013).
Yu. M. Harmaza, Candidate’s thesis in Biology (Minsk, 2011).
D. J. Eide, Biochim. Biophys. Acta 1763, 711 (2006).
K. R. Gee, Z. L. Zhou, D. Ton-That, et al., Cell Calcium 31 (5), 245 (2002).
A. R. Kay, BMC Physiol. 4 (4), 1 (2004).
D. Bratosin, L. Mitrofan, C. Palli, and J. Estaquier, Cytometry A 66A, 78 (2005).
G. L. Ellman, Arch. Biochem. Biophys. 82 (1), 70 (1959).
V. M. Moin, Lab. Delo 12, 724 (1986).
M. A. Korolyuk, L. I. Ivanova, I. G. Mayorova, and V. E. Tokoreva, Lab. Delo 1, 16 (1988).
O. G. Shevchenko and L. N. Shishkina, Usp. Sovrem. Biol. 134 (2), 133 (2014).
E. I. Slobozhanina, L. M. Luk’yanenko, and N. M. Kozlova, Biophysics (Moscow) 45 (2), 281 (2000).
A. Yesilkaya, A. Yegin, G. Yucel, et al., Int. J. Clin. Lab. Res. 26, 60 (1996).
M. G. Malakyan, S. A. Badjinyan, L. A. Vardevavyan, et al., Khim. Farm. Zh. 43 (1), 8 (2009).
T. Akerboom and H. Sies, in Glutathione: Metabolism and Physiological Functions, Ed. by E. J. Vina (CRC Press, Boston, 1990).
T. J. Simons, J. Membr. Biol. 123, 63 (1991).
E. I. Belevich, D. G. Kostin, and E. I. Slobozhanina, Izv. Nats. Akad. Nauk Belarusi, Ser. Biol. Nauk 2, 34 (2015).
M. Ilbert, P. C. Graf, and U. Jakob, Antioxid. Redox. Signal 8 (5–6), 835 (2006).
J. E. Raftos, S. Whillier, B. E. Chapman, and P. W. Kuchel, Int. J. Biochem. Cell Biol. 39 (9), 1698 (2007).
D. Yildiz and T. Bagdadioglu, Toxicol. Mech. Methods 14, 241 (2004).
N. G. Palmen and C. T. Evelo, Toxicol. In Vitro 10, 273 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © Yu.M. Harmaza, A.V. Tamashevski, Yu.S. Kanash, G.P. Zubritskaya, A.G. Kutko, E.I. Slobozhanina, 2016, published in Biofizika, 2016, Vol. 61, No. 6, pp. 1149–1158.
Rights and permissions
About this article
Cite this article
Harmaza, Y.M., Tamashevski, A.V., Kanash, Y.S. et al. The role of intracellular zinc in H2O2-induced oxidative stress in human erythrocytes. BIOPHYSICS 61, 950–958 (2016). https://doi.org/10.1134/S0006350916060087
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350916060087