Abstract
High efficiency of hybrid implants based on calcium-magnesium silicate ceramic, diopside, as a carrier of recombinant BMP-2 and xenogenic demineralized bone matrix (DBM) as a scaffold for bone tissue regeneration was demonstrated previously using the model of critical size cranial defects in mice. In order to investigate the possibility of using these implants for growing autologous bone tissue using in vivo bioreactor principle in the patient’s own body, effectiveness of ectopic osteogenesis induced by them in intramuscular implantation in mice was studied. At the dose of 7 μg of BMP-2 per implant, dense agglomeration of cells, probably skeletal muscle satellite precursor cells, was observed one week after implantation with areas of intense chondrogenesis, initial stage of indirect osteogenesis, around the implants. After 12 weeks, a dense bone capsule of trabecular structure was formed covered with periosteum and mature bone marrow located in the spaces between the trabeculae. The capsule volume was about 8-10 times the volume of the original implant. There were practically no signs of inflammation and foreign body reaction. Microcomputed tomography data showed significant increase of the relative bone volume, number of trabeculae, and bone tissue density in the group of mice with BMP-2-containing implant in comparison with the group without BMP-2. Considering that DBM can be obtained in practically unlimited quantities with required size and shape, and that BMP-2 is obtained by synthesis in E. coli cells and is relatively inexpensive, further development of the in vivo bioreactor model based on the hybrid implants constructed from BMP-2, diopside, and xenogenic DBM seems promising.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Repair of large bone defects caused by trauma, osteoporosis, tumor removal, or osteomyelitis is one of the most difficult tasks of reconstructive surgery [1, 2]. Gold standard of treatment in this case is autografting of the patient’s own bone tissue, usually taken from the ilium, which, however, has many problems, such as pain at the site of bone extraction, as well as limited volume of the autograft [3, 4]. A possible alternative is to grow autologous bone implants using the so-called in vivo bioreactor principle, where the patient’s own body serves as a bioreactor [5]. It is possible to grow bone of the required shape and size, for example, in the back or abdominal muscle, using a suitable scaffold and osteoinductive factors, and then transplant it into the right place. There are now more than a hundred publications describing ectopic bone formation in small (mice, rats, rabbits) and large (sheep, pigs, monkeys, goats, dogs) laboratory animals. There have also been published articles describing clinical cases, mainly concerning filling of large mandible defects in humans, based on this approach [6-13].
Successful development of a new in vivo bioreactor model requires selection of a combination of factors resulting in the mature bone formation at the ectopic site. Essential factors affecting the process are: choice of the laboratory animal, implantation model, scaffold material providing the required bone shape, osteogenesis inducer carrier, and osteogenesis inducer itself.
Mice are the most economical among the laboratory animals in terms of purchase price and maintenance costs. Therefore, their use seems reasonable for the initial testing of the model with the purpose of subsequent transfer to larger laboratory animals and humans.
Choice of the implantation model is extremely important. Currently, the most commonly used models are subcutaneous implantation, implantation into a muscle pocket (intramuscular implantation), and implantation into a renal capsule [14]. Intramuscular implantation model has several advantages over the other two models. In particular, it is almost as convenient in terms of surgical technique as subcutaneous implantation and much simpler than renal capsule implantation, while being fully adoptable to large laboratory animals and humans. Compared to the subcutaneous implantation, intramuscular implantation usually provides a more pronounced and earlier formation of bone tissue [15], which may be due to the better blood supply to muscle tissue, as well as presence of the satellite progenitor cells in the skeletal muscles capable of osteogenic differentiation under the action of appropriate inducers, such as bone morphogenetic proteins (BMPs) [14]. For example, in the study conducted on dogs and pigs, intramuscular implantation showed bone formation after 45 days, while bone formation through subcutaneous implantation took 60 days [16]. Using intramuscular implantation, bone tissue has been successfully grown to replace large jaw defects in humans. For example, in 2004 the paper describing growth of bone tissue in the muscle of a patient using autologous bone marrow and BMP-7 was published [7]. In fact, the ability of BMPs to induce ectopic osteogenesis was shown exactly with the model of intramuscular implantation [17].
An essential point when developing an in vivo bioreactor model is the choice of a scaffold that should serve as a matrix for autologous bone augmentation. Such material should be sufficiently rigid but elastic, mimeting properties of bone tissue. The material should also be biocompatible and bioresorbable, allowing its gradual replacement with the new bone tissue. One of the materials satisfying the required parameters is a highly purified xenogenic demineralized bone matrix (DBM) derived from bovine bone [18]. DBM has high osteoconductivity, it can be obtained in large quantities, implants of the required shape and volume can be produced from it, its mechanical properties and porosity, including microporosity, are maximally close to the human bone tissue; in addition, it is possible to obtain low immunogenic DBM with increased biocompatibility and reduced allergenicity due to reduced content of non-collagenous proteins [19, 20]. Limited osteoinductivity of DBM can be overcome by combining it with the exogenous osteogenesis inducers, such as recombinant bone morphogenetic protein 2 (BMP-2) [19, 21]. When introduced into the body, BMP-2 provides attraction of mesenchymal stem cells from the surrounding tissues and their osteogenic differentiation, and also has angiogenic properties, which is important for formation of the mature bone tissue with good blood supply [22]. DBM itself can act as a carrier of BMP-2, however, its sorption capacity is limited [23]. To be able to introduce large and controlled doses of BMP-2, as well as to provide more gradual protein release, hybrid implants were developed on the basis of DBM as a scaffold, in which calcium-magnesium silicate ceramics, diopside, suspended in hyaluronic acid (HA) performs the function of BMP-2 carrier. Diopside has very high sorption capacity in relation to BMP-2, many times exceeding capacity of hydroxyapatite. Such implants demonstrated high osteogenicity in the model of cranial defects of critical size in mice [23].
The aim of this study is to develop a model of ectopic trabecular bone tissue augmentation based on the in vivo bioreactor principle using intramuscular implantation of hybrid implants with xenogeneic DBM as a scaffold and calcium-magnesium silicate ceramic, diopside, as a carrier of recombinant BMP-2.
MATERIALS AND METHODS
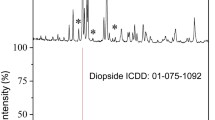
Preparation of diopside powder. Diopside powder (CaMgSi2O6) synthesized by solid-phase method according to the previously published method [24] with modifications [23] was used in the study. Briefly, egg shells were used as a source of calcium and rice husk as a source of silicon in a form of silicon dioxide obtained from a rice husk by heating. Crushed eggshells, silicon dioxide, and synthetic magnesium oxide (Ruskhim, Russia) were mixed at a weight ratio 1 : 2 : 1 in a Fritsch Pulverisette 5 planetary ball mill (Fritsch GmbH, Germany) and incubated at 1100°C for 6 h. The obtained diopside corresponded to the ICDD standard card no. 01-075-1092, with small contamination of the secondary phase, akermanite [23].
Preparation of demineralized bone matrix porous scaffolds for implantation. Highly purified xenogeneic DBM was obtained according to the previously described method from bovine femoral epiphyses [25]. Implants, which were discs with 4 mm in diameter and 1 mm thick, were made from bone plates using a custom metal perforator. For sterilization, DBM discs were incubated in 96% alcohol for 1 h, washed with a sterile PBS, and dried in a laminar flow cabinet with ultraviolet light overnight.
BMP-2 diopside powder saturation. Recombinant BMP-2 obtained by synthesis in Escherichia coli cells and purified as described previously [26] with a specific activity of 0.1 U/μg was immobilized on the diopside powder as described previously [23]. Briefly, 50 mg of diopside powder was incubated for 30 min in 10 mM sodium phosphate buffer (pH 5.5) with 50 mM NaCl, centrifuged, the solution was removed, the precipitate was suspended in the same solution containing 500 or 1000 µg BMP-2, and incubated for 2 h. The unbound protein was removed after centrifugation, and the precipitate was washed three times with the same protein-free buffer.
Preparation of samples for implantation. A 5% (w/v) suspension of diopside in HA, 50 mg of diopside powder (with or without 10 or 20 μg/mg BMP-2) in 250 μl of sterile saline was added to 750 ml of 2% HA (~100 kDa, Shanghai Macklin Biochemical Industry Co., Ltd., China) solution in saline and thoroughly mixed. Immediately before implantation, sterilized DBM discs were immersed in the pre-mixed suspension again. One disk absorbed approximately 7 μl of the suspension, and contained about 0.35 mg of diopside and 3.5 or 7 μg of BMP-2.
Implantation. We performed two experiments with male outbred mice of the ICR (CD-1) line, 38-47 days old. The first pilot experiment was performed with 24 mice, which were randomly divided into two groups with 12 mice in each. Group 1 was implanted with the DBM disks with a suspension of diopside powder without BMP-2, while group 2 was implanted with the disks with a suspension of diopside containing about 3.5 μg of BMP-2 per disk. Two identical implants were inserted in each animal – in the left and right femur. The second experiment was performed similarly to the first one, but 16 mice were used, divided into two groups of 8 mice in each. In the first group implants without protein were inserted, in the second group the implants with 7 µg of BMP-2 per disk were inserted. In both cases, the surgery was performed on a thermostated surgical table (Medax, Germany) at 37°C. After preparation of the surgical field, a skin incision was made in the caudal part of the thigh, then pockets were formed in the gluteal muscles by blunt dissection, after that implants were placed in them, tissues were sutured layer by layer with atraumatic suture material Mepfil no. 4/0 and treated with antibiotic “Terramycin” (Pfizer Animal Health, USA). In the first experiment, euthanasia by carbon dioxide was performed on the 7th, 24th, 63rd, and 84th day using three mice from each group. In the second experiment, two mice were euthanized on the 7th day and six mice from each group on the 84th day. Histological analysis of the samples extracted from the mice after euthanasia was performed 1 and 12 weeks after the beginning of the experiment; microcomputed tomography (microCT) was performed at the 12th week.
Histological analysis. Samples including implantation area and surrounding tissues were extracted from the femoral muscles, fixed with a buffered neutral formalin for 24 h, decalcified in a 14% EDTA solution, washed and passed through a battery of alcohol solutions of increasing concentration and embedded in paraffin. Sections of 3 μm thickness were stained with hematoxylin and eosin, Safranin O, hematoxylin, fast green FCF [27], and azan according to Heidengain [28].
Microcomputed tomography. MicroCT of the samples was performed ex vivo using a SkyScan 1172 tomograph (Bruker, USA) with NRecon and CTAn programs and selection of parameters allowing to distinguish mineralized bone tissue from other tissues.
Statistical processing of microCT data was performed using Statistica 12.0 software package (Statsoft, USA). Normality of distribution was tested using the Kolmogorov–Smirnov test. To assess significance of differences, the Mann–Whitney test was used. Differences were considered significant at p < 0.05.
RESULTS
Experimental design. In the pilot experiment on intramuscular implantation of DBM disks containing suspension of diopside powder in HA, BMP-2 was used in the experimental group in the amount of about 3.5 μg per implant, as in the previously described study on implantation into the cranial defects of mice [23], in which almost complete filling of the implant pores with newly formed bone tissue with bone marrow and remodeling of DBM matrix occurred in 9 weeks. At the 1st, 4th, 9th, and 12th weeks histological analysis revealed the picture of indirect ectopic osteogenesis with formation of cartilage tissue after 1 week. At later terms formation of a bone structure similar to the structure of trabecular bone observed in some animals by the 12th week appeared to be covered with a layer of bone tissue with periosteum (data are not shown). Based on the results of this experiment, it was decided to leave the general design of the main experiment unchanged (Fig. 1), but to double the amount of BMP-2, to perform histological analysis after the 1st and 12th week, and to perform micro-CT at the 12th week.
Evaluation of the results of intramuscular implantation of hybrid implants using microCT and histological analysis. The microCT data of the samples obtained 12 weeks after intramuscular implantation of the hybrid implants from DBM, HA, and diopside containing and not containing BMP-2 are presented in Fig. 2. Presence of diopside powder without BMP-2 in a number of samples induced formation of the volumetric mineralization grid corresponding in shape to the DBM macropores (Fig. 2a). However, according to the data of histological analysis, this was not accompanied by the appearance of newly formed bone tissue (Fig. 3a, 4-6, Fig. 4a, 4-6).
MicroCT results of the samples extracted 12 weeks after intramuscular implantation. a) Visualization of mineralization sites. b) Relative bone volume (BV/TV), number of trabeculae (Tb.N), trabecular thickness (Tb.Th), and bone mineral density (BMD) in the groups without (black rectangles) and with (empty rectangles) BMP-2. c) Sequential cross-sections in 3D visualization of the bone capsule microCT results from the group with BMP-2.
In the presence of BMP-2, a large, highly mineralized bone capsule (Fig. 2a) was observed in all samples 12 weeks after implantation according to the microCT data. It significantly exceeded the volume (8-10 times) of the original implant and had a pronounced trabecular structure (Fig. 2c). The parameters BV/TV (relative bone volume), Tb.Th (trabecular thickness), Tb.N (number of trabeculae), and BMD (bone mineral density) in the group with BMP-2 were significantly different (higher) in comparison with the group without BMP-2 (p < 0.05) (Fig. 2b).
According to the results of histological analysis in the group without BMP-2, the disk was surrounded by young granulation tissue one week after implantation (Fig. 3a, 1-3). Clusters of epithelioid cells were found around the diopside particles (Fig. 4a, 1-3). After 12 weeks, the disc was surrounded and filled with the well-organized fibrous tissue (Fig. 3a, 4-6). Around the diopside particles, presence of the giant multinucleated foreign body cells was observed, as well as collagenization and fibrosis of granulomatous nodules/foci of inflammation without signs of neoosteogenesis (Fig. 4a, 4-6).
Histological analysis of the samples obtained following implantation of the hybrid implants of DBM, diopside, and HA in the femoral muscle of mice without (a) and with (b) BMP-2. a, b) Hematoxylin and eosin staining on the left, Safranin O, hematoxylin, and FCF fast green staining in the center, and Heidenhain azan staining on the right. Magnification 10×. Designations: B, newly formed bone tissue, trabeculae; Ch, chondrocytes; F, fibrosis; G, granulation tissue; M, bone marrow; Mu, muscle tissue; O, osteoprogenitor cells; S, DBM scaffold. Black rectangles indicate areas corresponding to the areas shown at higher magnification in Fig. 4.
Histological analysis of the specimens obtained after implantation of hybrid implants of DBM, diopside, and HA in the femoral muscle of mice without (a) and with (b) BMP-2. a, b) Hematoxylin and eosin staining on the left, Safranin O, hematoxylin, and FCF fast green staining in the center, and Heidenhain azan staining on the right. Magnification 40×. Designations: A, adipocytes; B, newly formed bone tissue, trabeculae; Ch, chondrocytes; Os, cells with signs of osteocytes; Ob, osteoblasts; D, diopside; F, fibrosis; G, granulation tissue; M, bone marrow; Mu, muscle tissue; P, periosteum; S, DBM scaffold. Black arrow indicates osteocytes in the newly formed bone tissue, green arrows – epithelioid cells, yellow arrows – foreign body giant cells.
In the group with BMP-2, initial signs of osteogenesis appeared already one week after implantation: extensive zones of chondrogenesis (stained red with Safranin O) surrounded by numerous osteoprogenitor cells, probably formed from the pool of native skeletal progenitor cells, were observed around the implant (Fig. 3b, 1-3). At higher magnification, we observed residual chondroid cells replaced by the cells with osteocyte features, which were embedded in a matrix that was not stained with Safranin O (Fig. 4b, 1-3). After 12 weeks, the implant was surrounded by the bone capsule much larger than the size of implant, the entire volume of which was filled predominantly with bone marrow with adipocytes and thin bone trabeculae (Fig. 3b, 4-6). Outer layer of the capsule was represented by the dense newly formed bone covered with periosteum, which was clearly visible in the case of Heidenhain staining, where the collagen-containing structures are stained blue (Fig. 3b, 6; Fig. 4b, 6). At high magnification, one can observe inclusion of diopside particles in the newly formed bone tissue (Fig. 4b, 4 and 5), which can be distinguished from the demineralized bone of the DBM scaffold by the presence of osteocytes.
DISCUSSION
According to the results of microCT of the implantation area, pronounced tissue mineralization is observed both in the case of samples with and without BMP-2 (Fig. 2). In the absence of BMP-2 12 weeks after implantation mineralized areas are located in the open pores of the DBM scaffold forming a characteristic grid (Fig. 2a), however, it is not accompanied by the appearance of newly formed bone tissue – histological sections show the presence of fibrous tissue in the implant pores (Fig. 4a, 4-6). The observed areas with X-ray contrast apparently correspond to calcium deposits on the diopside particles, which appear as a result of biomineralization characteristic for diopside – formation of apatite surface layer during incubation in the body or in SBF (simulated body fluid) [29]. Thus, diopside, having the ability to biomineralize, is, nevertheless, not able to induce osteogenesis by itself.
In the presence of BMP-2, formation of the closed bone capsule covered with a periosteum is observed, with a developed system of trabeculae built from the newly formed bone tissue with gaps between them filled with mature bone marrow. Hence, the implant designed in this study leads to formation of the fully formed autogenous bone structure ready for autotransplantation when introduced into the muscle, which testifies in favor of the optimal selection of the implant components, in particular, the carrier for BMP-2. Choice of the carrier for BMP-2 is vital for the successful formation of ectopic bone tissue. Thus, in the case of using β-tricalcium phosphate as a carrier for BMP-2, ectopic bone formation was observed during intramuscular implantation in rats [30]; at the same time, when hydroxyapatite was used, only additional introduction of collagen into the implant resulted in bone tissue formation [17]. Pronounced biomineralization ability of diopside may lead to enhanced osteogenesis in the vicinity of diopside particles in the presence of osteoinductor due to creation of high local concentration of calcium ions at the implantation site. This may be an additional factor providing high level of ectopic osteogenesis in this experiment, as well as orthotopic osteogenesis in the earlier experiment on implantation of DBM and diopside with BMP-2 into the cranial defects of critical size in mice [23]. The fact that in the absence of BMP-2 in the implantation area there is a moderately pronounced inflammatory and cellular reaction to implantation, which does not exceed the usual reaction to the introduction of osteoplastic materials containing DBM and ceramic particles, also indicates that the use of diopside as a carrier of BMP-2 is promising. Inflammation is significantly reduced in the presence of BMP-2.
We were interested not only in the possibility of obtaining ectopic bone, but also in characterizing early and late stages of osteogenesis, including the structure and quality of the obtained bone tissue. In this regard, micro-CT and detailed histological analysis with three stains at different magnifications were performed, which allowed us to evaluate at two time periods both general picture observed during implantation and phenomena occurring at the cellular level. The pattern observed in the presence of BMP-2 corresponds to indirect osteogenesis taking place with early cartilage formation. Structures similar to bone capsule with a trabecular structure (similar to cortical and cancellous bone) formed at the late stages are quite rarely observed in the studies on ectopic osteogenesis. This is probably due to the lower osteogenicity of the used materials, use of more rigid models of ectopic osteogenesis, and the fact that in many cases, researchers are interested in the very fact of the possibility of ectopic osteogenesis using certain materials, and duration of the experiment is usually relatively short.
It is interesting to trace the fate of the xenogenic bone matrix acting as a scaffold in the used implants. After 12 weeks, the original DBM scaffold is still present inside the capsule; however, its upper layers are remodeled with formation of the new bone tissue with lacunae containing osteocytes. Relative thickness of the DBM scaffold trabeculae increases significantly by the week 12, which is clearly visible in Fig. 3b presenting a general view of the formed bone capsule. However, complete remodeling of the DBM bone matrix did not occur 12 weeks after implantation in our experiment. At the same time, in the case of introduction of a similar implant into the critical-sized cranial defects in mice, after 9 weeks from the beginning of the experiment and with half the amount of BMP-2, almost complete remodeling of DBM with formation of the newly formed bone tissue with osteocytes included in it was observed [23]. Different rates of remodeling may be due to various reasons, in particular, variations in the intensity and pathways of osteogenesis due to different origins of osteoprogenitor cells: in the case of orthotopic osteogenesis they are mesenchymal stromal stem cells from the bone marrow of the maternal bone and periosteum, while in the case of ectopic osteogenesis in the muscle pocket they are satellite cells of skeletal muscle progenitors [14]. It is of interest to investigate the fate of such implants at later terms. A complete remodeling with replacement of the xenogenic bone matrix with autogenous bone using the bone capsule as a source of osteoprogenitor cells of the periosteum could be presumed to occur at the later stages.
Recombinant BMP-2 used in the experiment was synthesized in the E. coli cells [26]. High osteogenic activity of this protein has been shown when used together with various natural and synthetic materials [21, 31-36]. This BMP-2 variant is able to spontaneously sorb onto a diopside powder in amounts greater than 150 μg/mg and show continuous release of the protein over several days [23], making this osteoinducer-carrier combination very attractive for the use in bone defect regeneration experiments. In this study, the selected dose of BMP-2 (7 μg per implant) facilitated effective ectopic osteogenesis with trabecular bone formation. It should be noted that usually the dose of BMP-2 used in the experiments on ectopic osteogenesis, especially in large animals, is significantly higher [17, 35]. Moreover, as a rule, commercial BMP-2 synthesized in Chinese hamster cells is used, which obviously determines very high cost of the autologous bone production based on the principle of in vivo bioreactor and limit applicability of this approach. The use of a cheaper but no less active BMP-2 synthesized in E. coli cells should contribute to a wider acceptance of this method.
CONCLUSION
In the present work, an experiment on ectopic osteogenesis induced by hybrid implants consisting of spongy DBM filled with a suspension of diopside powder with BMP-2 in HA was performed using the model of intramuscular implantation in mice. After 12 weeks, rounded bone capsules of trabecular structure filled with mature bone marrow and covered with bone with periosteum several times larger in volume than the original implant were formed at the implantation site. Histological examination at an early time point, 1 week after implantation, confirmed the mechanism of indirect osteogenesis with intermediate cartilage formation. The proposed approach for ectopic bone augmentation using particles of calcium-magnesium silicate ceramic, diopside, as BMP-2 carrier is new; advantage of this approach is the possibility of very high and adjustable loading capacity of BMP-2 into the implant. Implants based on DBM and diopside saturated with BMP-2 can be used to develop approaches to obtain autologous bone tissue using the in vivo bioreactor principle for repairing large trabecular bone defects. Development of other types of hybrid implants based on diopside as a carrier of BMP-2 including, for example, various hydrogels and/or porous scaffolds of polymeric nature also seems promising.
Abbreviations
- BMP-2:
-
bone morphogenetic protein-2
- DBM:
-
demineralized bone matrix
- HA:
-
hyaluronic acid
References
Tan, W., Gao, C., Feng, P., Liu, Q., Liu, C., Wang, Z., Deng, Y., and Shuai, C. (2021) Dual-functional scaffolds of poly(L-lactic acid)/nanohydroxyapatite encapsulated with metformin: Simultaneous enhancement of bone repair and bone tumor inhibition, Mater. Sci. Eng. C Mater. Biol. Appl., 120, 111592, https://doi.org/10.1016/j.msec.2020.111592.
Xu, B., Zheng, P., Gao, F., Wang, W., Zhang, H., Zhang, X., Feng, X., and Liu, W. A. (2017) Mineralized high strength and tough hydrogel for skull bone regeneration, Adv. Funct. Mater., 27, 1604327, https://doi.org/10.1002/adfm.201604327.
Farokhi, M., Mottaghitalab, F., Shokrgozar, M. A., Ou, K. L., Mao, C., and Hosseinkhani, H. (2016) Importance of dual delivery systems for bone tissue engineering, J. Control. Release, 225, 152-169, https://doi.org/10.1016/j.jconrel.2016.01.0334.
Norbertczak, H. T., Fermor, H. L., Edwards, J. H., Rooney, P., Ingham, E., and Herbert, A. (2022) Decellularised human bone allograft from different anatomical sites as a basis for functionally stratified repair material for bone defects, J. Mech. Behav. Biomed. Mater., 125, 104965, https://doi.org/10.1016/j.jmbbm.2021.104965.
Huang, R. L., Kobayashi, E., Liu, K., and Li, Q. (2016) Bone graft prefabrication following the in vivo bioreactor principle, EBioMedicine, 12, 43-54, https://doi.org/10.1016/j.ebiom.2016.09.016.
Orringer, J. S., Shaw, W. W., Borud, L. J., Freymiller, E. G., Wang, S. A., and Markowitz, B. L. (1999) Total mandibular and lower lip reconstruction with a prefabricated osteocutaneous free flap, Plast. Reconstr. Surg., 104, 793-797, https://doi.org/10.1097/00006534-199909030-00028.
Warnke, P. H., Springer, I. N., Wiltfang, J., Acil, Y., Eufinger, H., Wehmöller, M., Russo, P. A., Bolte, H., Sherry, E., Behrens, E., and Terheyden, H. (2004) Growth and transplantation of a custom vascularised bone graft in a man, Lancet, 364, 766-770, https://doi.org/10.1016/S0140-6736(04)16935-3.
Warnke, P., Wiltfang, J., Springer, I., Acil, Y., Bolte, H., Kosmahl, M., Russo, P., Sherry, E., Lutzen, U., and Wolfart, S. (2006) Man as living bioreactor: fate of an exogenously prepared customized tissue-engineered mandible, Biomaterials, 27, 3163-3167, https://doi.org/10.1016/j.biomaterials.2006.01.050.
Heliotis, M., Lavery, K. M., Ripamonti, U., Tsiridis, E., and di Silvio, L. (2006) Transformation of a prefabricated hydroxyapatite/osteogenic protein-1 implant into a vascularised pedicled bone flap in the human chest, Int. J. Oral Maxillofac, Surg., 35, 265-269, https://doi.org/10.1016/j.ijom.2005.07.013.
Cheng, M., Brey, E. M., Ulusal, B. G., and Wei, F. (2006) Mandible augmentation for osseointegrated implants using tissue engineering strategies, Plast. Reconstr. Surg., 118, 1e-4e, https://doi.org/10.1097/01.prs.0000221120.11128.1a.
Mesimäki, K., Lindroos, B., Törnwall, J., Mauno, J., Lindqvist, C., Kontio, R., Miettinen, S., and Suuronen, R. (2009) Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells, Int. J. Oral Maxillofac. Surg., 38, 201-209, https://doi.org/10.1016/j.ijom.2009.01.001.
Kokemueller, H., Spalthoff, S., Nolff, M., Tavassol, F., Essig, H., Stuehmer, C., Bormann, K. H., Rücker, M., and Gellrich, N. C. (2010) Prefabrication of vascularized bioartificial bone grafts in vivo for segmental mandibular reconstruction: experimental pilot study in sheep and first clinical application, Int. J. Oral Maxillofac Surg., 39, 379-387, https://doi.org/10.1016/j.ijom.2010.01.010.
Horch, R. E., Beier, J. P., Kneser, U., and Arkudas, A. (2014) Successful human long-term application of in situ bone tissue engineering, J. Cell. Mol. Med., 18, 1478-1485, https://doi.org/10.1111/jcmm.12296.
Scott, M. A., Levi, B., Askarinam, A., Nguyen, A., Rackohn, T., Ting, K., Soo, C., and James, A. W. (2012) Brief review of models of ectopic bone formation, Stem Cells Dev., 21, 655-667, https://doi.org/10.1089/scd.2011.0517.
Habibovic, P., and de Groot, K. (2007) Osteoinductive biomaterials – properties and relevance in bone repair, J. Tissue Engin. Regenerat. Med., 1, 25-32, https://doi.org/10.1002/term.5.
Yang, Z., Yuan, H., Tong, W., Zou, P., Chen, W., and Zhang, X. (1996) Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: variability among different kinds of animals, Biomaterials, 17, 2131-2137, https://doi.org/10.1016/0142-9612(96)00044-0.
Takaoka, K., Nakahara, H., Yoshikawa, H., Masuhara, K., Tsuda, T., and Ono, K. (1988) Ectopic bone induction on and in porous hydroxyapatite combined with collagen and bone morphogenetic protein, Clin. Orthopaed. Rel. Res., 234, 250-254.
Zhang, H., Yang, L., Yang, X. G., Wang, F., Feng, J. T., Hua, K. C., Li, Q., and Hu, Y. C. (2019) Demineralized bone matrix carriers and their clinical applications: an overview, Orthopaedic Surg., 11, 725-737, https://doi.org/10.1111/os.12509.
Bartov, M. S., Gromov, A. V., Poponova, M. S., Savina, D. M., Nikitin, K. E., Grunina, T. M., Manskikh, V. N., Gra, O. A., Lunin, V. G., Karyagina, A. S., and Gintsburg, A. L. (2016) Modern approaches to research of new osteogenic biomaterials on the model of regeneration of cranial critical-sized defects in rats, Bull. Exp. Biol. Med., 162, 273-276, https://doi.org/10.1007/s10517-017-3693-2.
Gromov, A. V., Nikitin, K. E., Karpova, T. A., Zaitsev, V. V., Sidorova, E. I., Andreeva, E. V., Bartov, M. S., Mishina, D. M., Subbotina, M. E., Shevlyagina, N. V., Sergienkov, M. A., Soboleva, L. A., Kotnova, A. P., Sharapova, N. E., Semikhin, A. S., Didenko, L. V., Karyagina, A. S., and Lunin, V. G. (2012) Development of a technique for obtaining osteoplastic material based on demineralized bone matrix with a maximum content of native bone growth factors, Biotechnol. Russ., 5, 66-75.
Gromov, A. V., Bartov, M. S., Orlova, P. A., Manskikh, V. N., Krivozubov, M. S., Grunina, T. M., Manukhina, M. S., Strukova, N. V., Nikitin, K. E., Lunin, V. G., Karyagina, A. S., and Gintsburg, A. L. (2019) Combined effect of bone morphogenetic protein-2 and erythropoietin on regeneration of cranial bone defects in mice, Bull. Exp. Biol. Med., 167, 408-412, https://doi.org/10.1007/s10517-019-04538-5.
Gromov, A. V., Poponova, M. S., and Karyagina, A. S. (2020) Recombinant human bone growth factor BMP-2 produced in Escherichia coli, Part 1: from protein purification to experimental models for efficacy research, Mol. Genet. Microbiol. Virol., 35, 22-31, https://doi.org/10.3103/S0891416820010036.
Karyagina, A. S., Orlova, P. A., Poponova, M. S., Bulygina, I. N., Choudhary, R., Zhulina, A. V., Grunina, T. M., Nikitin, K. E., Strukova, N. V., Generalova, M. S., Ryazanova, A. V., Kovalyova, P. A., Zimina, A. I., Lukinova, E. M., Plakhotniuk, E. D., Kirsanova, M. A., Kolesnikov, E. A., Zakharova, E. V., Manskikh, V. N., Senatov, F. S., and Gromov, A. V. (2022) Hybrid implants based on calcium-magnesium silicate ceramics diopside as a carrier of recombinant BMP-2 and demineralized bone matrix as a scaffold: dynamics of reparative osteogenesis in a mouse craniotomy model, Biochemistry (Moscow), 87, 1277-1291, https://doi.org/10.1134/S0006297922110074.
Choudhary, R., Venkatraman, S. K., Bulygina, I., Senatov, F., Kaloshkin, S., Anisimova, N., Kiselevskiy, M., Knyazeva, M., Kukui, D., Walther, F., and Swamiappan, S. (2021) Biomineralization, dissolution and cellular studies of silicate bioceramics prepared from eggshell and rice husk, Mater. Sci. Engin. C, 118, 111456, https://doi.org/10.1016/j.msec.2020.111456.
Plantz, M. A., Minardi, S., Lyons, J. G., Greene, A. C., Ellenbogen, D. J., Hallman, M., Yamaguchi, J. T., Jeong, S., Yun, C., Jakus, A. E., Blank, K. R., Havey, R. M., Muriuki, M., Patwardhan, A. G., Shah, R. N., Hsu, W. K., Stock, S. R., and Hsu, E. L. (2021) Osteoinductivity and biomechanical assessment of a 3D printed demineralized bone matrix-ceramic composite in a rat spine fusion model, Acta Biomater., 127, 146-158, https://doi.org/10.1016/j.actbio.2021.03.060.
Karyagina, A. S., Boksha, I. S., Grunina, T. M., Demidenko, A. V., Poponova, M. S., Sergienko, O. V., Lyashchuk, A. M., Galushkina, Z. M., Soboleva, L. A., Osidak, E. O., Bartov, M. S., Gromov, A. V., and Lunin, V. G. (2017) Two variants of recombinant human bone morphogenetic protein 2 (rhBMP-2) with additional protein domains: synthesis in an Escherichia coli heterologous expression system, Biochemistry (Moscow), 82, 613-624, https://doi.org/10.1134/S0006297917050091.
Bryan, J. H. D. (1954) Differential staining with a mixture of safranin and fast green FCF, Stain Technol., 30, 153-157.
Heidenhain, M. (1905) Zeitschrift fur wissenschaftliche Mikroskopie und fur mikroskopische Technik, S. Hirzel, Leipzig, 22, pp. 339.
Wu, C., and Chang, J. (2013) A review of bioactive silicate ceramics, Biomed. Mater., 8, 032001, https://doi.org/10.1088/1748-6041/8/3/032001.
Oda, S., Kinoshita, A., Higuchi, T., Shizuya, T., and Ishikawa, I. (1997) Ectopic bone formation by biphasic calcium phosphate (BCP) combined with recombinant human bone morphogenetic protein-2 (rhBMP-2), J. Med. Dent. Sci., 44, 53-62.
Zimina, A., Senatov, F., Choudhary, R., Kolesnikov, E., Anisimova, N., Kiselevskiy, M., Orlova, P., Strukova, N., Generalova, M., Manskikh, V., Gromov, A., and Karyagina, A. (2020) Biocompatibility and physico-chemical properties of highly porous PLA/HA scaffolds for bone reconstruction, Polymers, 12, 2938, https://doi.org/10.3390/polym12122938.
Senatov, F., Gulbanu, A., Orlova, P., Bartov, M., Grunina, T., Kolesnikov, E., Maksimkin, A., Kaloshkin, S., Poponova, M., Nikitin, K., Krivozubov, M., Strukova, N., Manskikh, V., Anisimova, N., Kiselevskiy, M., Scholz, R., Knyazeva, M., Walther, F., Lunin, V., Gromov, A., and Karyagina, A. (2020) Biomimetic UHMWPE/HA scaffolds with rhBMP-2 and erythropoietin for reconstructive surgery, Mater. Sci. Eng. C, 111, 110750, https://doi.org/10.1016/j.msec.2020.110750.
Senatov, F., Maksimkin, A., Chubrik, A., Kolesnikov, E., Orlova, P., Krivozubov, M., Nikitin, K., Gromov, A., and Karyagina, A. (2021) Osseointegration evaluation of UHMWPE and PEEK-based scaffolds with BMP-2 using model of critical-size cranial defect in mice and push-out test, J. Mech. Behav. Biomed. Mater., 119, 104477, https://doi.org/10.1016/j.jmbbm.2021.104477.
Senatov, F., Zimina, A., Chubrik, A., Kolesnikov, E., Permyakova, E., Voronin, A., Poponova, M., Orlova, P., Grunina, T., Nikitin, K., Krivozubov, M., Strukova, N., Generalova, M., Ryazanova, A., Manskikh, V., Lunin, V., Gromov, A., and Karyagina, A. (2022) Effect of recombinant BMP-2 and erythropoietin on osteogenic properties of biomimetic PLA/PCL/HA and PHB/HA scaffolds in critical-size cranial defects model, Mater. Sci. Eng. C Mater. Biol. Appl., 135, 112680, https://doi.org/10.1016/j.msec.2022.112680.
Karpov, T. E., Peltek, O. O., Muslimov, A. R., Tarakanchikova, Y. V., Grunina, T. M., Poponova, M. S., Karyagina, A. S., Chernozem, R. V., Pariy, I. O., Mukhortova, Y. R., Zhukov, M. V., Surmeneva, M. A., Zyuzin, M. V., Timin, A. S., and Surmenev, R. A. (2020) Development of optimized strategies for growth factor incorporation onto electrospun fibrous scaffolds to promote prolonged release, ACS Appl. Mater. Interfaces, 12, 5578-5592, https://doi.org/10.1021/acsami.9b20697.
Chubrik, A., Senatov, F., Kolesnikov, E., Orlova, P., Poponova, M., Grunina, T., Bartov, M., Nikitin, K., Krivozubov, M., Generalova, M., Manskikh, V., Lunin, V., Gromov, A., and Karyagina, A. (2020) Highly porous PEEK and PEEK/HA scaffolds with Escherichia coli-derived recombinant BMP-2 and erythropoietin for enhanced osteogenesis and angiogenesis, Polym. Test., 87, 106518, https://doi.org/10.1016/j.polymertesting.2020.
Acknowledgments
The authors thank Rajan Chouldhari and Inna Bulygina for providing diopside powder, Maria Generalova and Anna Ryazanova for assistance in implantation. Synthesis of diopside was carried out at the National University of Science and Technology (MISIS), and a number of BMP-2 isolation stages were carried out at the A. N. Belozersky Research Institute of Physico-Chemical Biology, Lomonosov Moscow State University, and at the All-Russia Research Institute of Agricultural Biotechnology.
Funding
The study was financially supported by the Russian Science Foundation (project no. 22-15-00216).
Author information
Authors and Affiliations
Contributions
A.S.K. concept and supervision of the work, writing of the article; P.A.O., A.V.Zh., M.S.K., T.M.G., N.V.S. conducting experiments; P.A.O., M.S.K., A.V.G. writing fragments of the article, preparing illustrations; A.S.K., V.N.M., F.S.S., A.V.G. discussing the results, editing the text of the article.
Corresponding authors
Ethics declarations
The authors declare no conflict of interest in financial or any other spheres. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karyagina, A.S., Orlova, P.A., Zhulina, A.V. et al. Hybrid Implants Based on Calcium-Magnesium Silicate Ceramic Diopside as a Carrier of Recombinant BMP-2 and Demineralized Bone Matrix as a Scaffold: Ectopic Osteogenesis in Intramuscular Implantation in Mice. Biochemistry Moscow 88, 1116–1125 (2023). https://doi.org/10.1134/S0006297923080060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923080060