Abstract
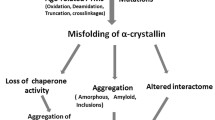
This issue of Biochemistry (Moscow) is dedicated to the role of protein misfolding and aggregation in cataract development. In fact, many genetic mutations or chemical and physical deleterious factors can initiate alterations in the macrostructural order and proper folding of eye lens proteins, which in some cases result in the formation of large light-scattering aggregates, affecting the quality of vision and making lens more prone to cataract development. Diabetes mellitus, which is associated with oxidative stress and mass production of highly reactive compounds, can accelerate unfolding and aggregation of eye lens proteins. This journal issue contains reviews and research articles that describe the destructive effects of mutations and highly reactive metabolites on the structure and function of lens crystallin proteins, as well important molecules in the lens’s natural defense system involved in protection against deleterious effects of the physical and chemical factors.
Similar content being viewed by others
References
Delaye, M. (1983) Short-range order of crystallin proteins accounts for eye lens transparency, Nature, 302, 415-417.
Bloemendal, H. (1977) The vertebrate eye lens, Science, 197, 127-138.
Lampi, K. J., Ma, Z., Shih, M., Shearer, T. R., Smith, J. B., et al. (1997) Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens, J. Biol. Chem., 272, 2268-2275.
Benedek, G. B. (1971) Theory of transparency of the eye, Appl. Opt., 10, 459-473.
Bera, S., and Abraham, E. C. (2002) The αA-crystallin R116C mutant has a higher affinity for forming heteroaggregates with αB-crystallin, Biochemistry, 41, 297-305.
Basha, E., O’Neill, H., and Vierling, E. (2012) Small heat shock proteins and α-crystallins: Dynamic proteins with flexible functions, Trends Biochem. Sci., 37, 106-117.
Bron, A. J., Vrensen, G., Koretz, J., Maraini, G., and Harding, J. (2000) The ageing lens, Opthalmologica, 214, 86-104.
Takemoto, L. J., and Ponce, A. A. (2006) Decreased association of aged alpha crystallins with gamma crystallins, Exp. Eye Res., 83, 793-797.
Treweek, T. M., Rekas, A., Lindner, R. A., Walker, M. J., Aquilina, J. A., et al. (2005) R120G αB-crystallin promotes the unfolding of reduced α-lactalbumin and is inherently unstable, FEBS J., 272, 711-724.
Dubin, R. A., Wawrousek, E. F., and Piatigorsky, J. (1989) Expression of the Murine αB-crystallin gene is not restricted to the lens, Mol. Cell Biol., 9, 1083-1091.
Sax, C. M., and Piatigorsky, J. (1994) Expression of the alpha-crystallin/small heat-shock protein/molecular chaperone genes in the lens and other tissues, Adv. Enzymol. Relat. Areas Mol. Biol., 69, 155-201.
Koletsa, T., Stavridi, F., Bobos, M., Kostopoulos, I., Kotoula, V., et al. (2014) alphaB-crystallin is a marker of aggressive breast cancer behavior but does not independently predict for patient outcome: A combined analysis of two randomized studies, BMC Clin. Pathol., 14, 1-13.
Rajasekaran, N. S., Connell, P., Christians, E. S., Yan, L., Taylor, R. P., et al. (2007) Human alphaB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice, Cell, 130, 427-439.
Simon, S., Fontaine, J., Martin, J. L., Sun, X., Hoppe, A. D., et al. (2007) Myopathy-associated alphaB-crystallin mutants abnormal phosphorylation, intracellular location, and interactions with other small heat shock proteins, J. Biol. Chem., 282, 34276-34287.
Avliyakulov, N. K., Rajavel, K. S., Minh, K., Haykinson, M. J., and Pope, W. B. (2014) C-terminally truncated form of alphaB-crystallin is associated with IDH1 R132H mutation in anaplastic astrocytoma, J. Neurooncol., 117, 53-65.
Boelens, W. C. (2014) Cell biological roles of αB-crystallin, Prog. Biophys. Mol. Biol., 115, 3-10.
Wistow, G., and Kim, H. (1991) Lens protein expression in mammals: Taxon-specificity and the recruitment of crystallins, J. Mol. Evol., 32, 262-269.
Horwitz, J. (1992) a-Crystallin can function as a molecular chaperone, Proc. Natl. Acad. Sci. USA, 89, 10449-10453.
Andley, U. P. (2007) Crystallins in the eye: Function and pathology, Prog. Retin. Eye Res., 26, 78-98.
Van Montfort, R., Slingsby, C., and Vierlingt, E. (2001) structure and function of the small heat shock protein/α-crystallin family of molecular chaperones, Adv. Protein Chem., 59, 105-156.
Pasupuleti, N., Matsuyama, S., Voss, O., Doseff, A. I., Song, K., et al. (2010) The anti-apoptotic function of human αA-crystallin is directly related to its chaperone activity, Cell Death Dis., 1, e31.
Roskamp, K. W., Paulson, C. N., Brubaker, W. D., and Martin, R. W. (2020) Function and aggregation in structural eye lens crystallins, Acc. Chem. Res., 53, 863-874.
Clark, A. R., Lubsen, N. H., and Slingsby, C. (2012) sHSP in the eye lens: Crystallin mutations, cataract and proteostasis, Int. J. Biochem. Cell Biol., 44, 1687-1697.
Yousefi, R., Javadi, S., Amirghofran, S., Oryan, A., and Moosavi-Movahedi, A. A. (2016) Assessment of structure, stability and aggregation of soluble lens proteins and alpha-crystallin upon non-enzymatic glycation: The pathomechanisms underlying cataract development in diabetic patients, Int. J. Biol. Macromol., 82, 328-338.
Zafaranchi, S., Khoshaman, K., Masoudi, R., Hemmateenejad, B., and Yousefi, R. (2017) The structural alteration and aggregation propensity of glycated lens crystallins in the presence of calcium: Importance of lens calcium homeostasis in development of diabetic cataracts, Spectrochim. Acta Part A Mol. Biomol. Spectrosc., 170, 174-183.
Dimauro, I., Antonioni, A., Mercatelli, N., and Caporossi, D. (2018) The role of αB-crystallin in skeletal and cardiac muscle tissues, Cell Stress Chaperones, 23, 491-505.
Shiels, A., Hejtmancik, J. F., Sciences, V., and Branch, V. F. (2017) Mutations and mechanisms in congenital and age-related cataracts, Exp. Eye Res., 156, 95-102.
Phadte, A. S., Sluzala, Z. B., and Fort, P. E. (2021) Therapeutic potential of α-crystallins in retinal neurodegenerative diseases, Antioxidants, 10, 1-13.
Pescosolido, N., Barbato, A., Giannotti, R., Komaiha, C., and Lenarduzzi, F. (2016) Age-related changes in the kinetics of human lenses: Prevention of the cataract, Int. J. Ophthalmol., 9, 1506.
Varma, S. D., Kovtun, S., and Hegde, K. R. (2011) Role of UV irradiation and oxidative stress in cataract formation. Medical prevention by nutritional antioxidants and metabolic agonists, Eye Contact Lens, 37, 233-245.
Linetsky, M., Shipova, E., Cheng, R., and Ortwerth, B. J. (2008) Glycation by ascorbic acid oxidation products leads to the aggregation of lens proteins, Biochim. Biophys. Acta, 1782, 22-34.
Pande, A., Mokhor, N., Pande, J., and States, U. (2018) Deamidation of human γS-crystallin increases attractive protein interactions: Implications for cataract, Biochemistry, 54, 4890-4899.
Gong, X., Li, E., Klier, G., Huang, Q., Wu, Y., et al. (1997) Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice, Cell, 91, 833-843.
Kashani, M. R., Yousefi, R., Akbarian, M., Alavianmehr, M. M., and Ghasemi, Y. (2016) Structure, chaperone activity, and aggregation of wild type and R12C mutant αB crystallins in the presence of thermal stress and calcium ion – implications for role of calcium in cataract pathogenesis, Biochemistry, 81, 122-134.
Ghahramani, M., Yousefi, R., Khoshaman, K., Sasan, S., and Kurganov, B. I. (2016) Evaluation of structure, chaperone-like activity and protective ability of peroxynitrite modified human alpha-Crystallin subunits against copper-mediated ascorbic acid oxidation, Int. J. Biol. Macromol., 87, 208-221.
Calva, J. A. D., Vázquez, M. L. P., and King, J. A., and Quintanar, L. (2018) Mercury-induced aggregation of human lens γ‑crystallins reveals a potential role in cataract disease, J. Biol. Inorg. Chem., 23, 1105-1118.
Kempka, K., Kaminski, P., Malukiewicz, G., Bogdzinska, M., and Florczak, S. (2018) Initial pro-antioxidant reactions in the patients suffering from cataract in the interactions with cadmium and lead, World Sci., 108, 195-206.
Kyselova, Z., Stefek, M., and Bauer, V. (2004) Pharmacological prevention of diabetic cataract, J. Diabetes Complicat., 18, 129-140.
Moreau, K. L., and King, J. A. (2012) Protein misfolding and aggregation in cataract disease and prospects for prevention, Trends Mol. Med., 18, 273-282.
Kato, K., Ito, H., Kamei, K., and Iwamoto, I. (1998) Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo, Cell Stress Chaperones, 3, 152.
Khoshaman, K., Yousefi, R., and Moosavi-Movahedi, A. A. (2017) Protective role of antioxidant compounds against peroxynitrite-mediated modification of R54C mutant a A-crystallin, Arch. Biochem. Biophys., 629, 43-53.
Jara, O., Minogue, P. J., Berthoud, V. M., and Beyer, E. C. (2018) Chemical chaperone treatment improves levels and distributions of connexins in Cx50D47A mouse lenses, Exp. Eye Res., 175, 192-198.
Lian, R. R., and Afshari, N. A. (2020) The quest for homeopathic and nonsurgical cataract treatment, Curr. Opin. Ophthalmol., 31, 61-66.
Liu, Z., Wang, R., Lin, H., and Liu, Y. (2020) Lens regeneration in humans: using regenerative potential for tissue repairing, Ann. Transl. Med., 8, 1-17.
Mikhaylova, V., Eronina, T., and Kurganov, B. (2021) The effect of chemical chaperones on test systems with different kinetic regime of aggregation, FEBS Open Bio, 11, 170-170.
Ghahramani, M., Yousefi, R., Krivandin, A., Muranov, K., Kurganov, B., et al. (2020) Kinetic data analysis of chaperone-like activity of Wt, R69C and D109H αB-crystallins, Data in Brief, 28, 104922.
Kurganov, B. I. (2017) Quantification of anti-aggregation activity of chaperones, Int. J. Biol. Macromol., 100, 104-117.
Kurganov, B. I. (2015) Selection of test systems for estimation of anti-aggregation activity of molecular chaperones, Biochem. Anal. Biochem., 4, 1.
Kurganov, B. I. (2014) Estimation of chaperone-like activity using test systems based on protein amyloid aggregation, Biochem. Anal. Biochem., 4, https://doi.org/10.4172/2161-1009.1000160.
Borzova, V. A., Markossian, K. A., Kara, D. A., Chebotareva, N. A., Makeeva, V. F., et al. (2013) Quantification of anti-aggregation activity of chaperones: A test-system based on dithiothreitol-induced aggregation of bovine serum albumin, PLoS One, 8, e74367.
Chebotareva, N. A., Eronina, T. B., Mikhaylova, V., Roman, S. G., Tugaeva, K. V., et al. (2022) Effect of trehalose on oligomeric state and anti-aggregation activity of αB-Crystallin, Biochemistry (Moscow), 87, 121-130.
Sharma, K. K., and Santhoshkumar, P. (2009) Lens aging: Effects of crystallins, Biochim. Biophys. Acta, 1790, 1095-1108.
Muranov, K. O., and Ostrovsky, M. O. (2022) Biochemistry of Eye Lens in the Norm and in Cataractogenesis, Biochemistry (Moscow), 87, 106-120.
Moghadam, S. S., Oryan, A., Kurganov, B. I., Tamaddon, A. M., Alavianehr, M. M., et al. (2017) The structural damages of lens crystallins with peroxynitrite and methylglyoxal, two causetive players in diabetic complications and preventive role of lens antioxidant components, Int. J. Biol. Macromol., 103, 74-88.
Stitt, A. (2005) The maillard reaction in eye disease, Ann. N. Y. Acad. Sci., 1043, 582-597.
Patel, D. K., Prasad, S. K., Kumar, R., and Hemalatha, S. (2011) Cataract: A major secondary complication of diabetes, its epidemiology and an overview on major medicinal plants screened for anticataract activity, Asian Pac. J. Trop. Dis., 1, 323-329.
Kumar, C. U., Suryavanshi, U., Sontake, V., Reddy, P. Y., Sankhala, R. S., et al. (2022) Effects of sorbitol on alpha-crystallin structure and function, Biochemistry (Moscow), 87, 131-140.
Moghadam, S.S., Ghahramani, M., Khoshaman, K., Oryan, A., Moosavi-Movahedi, A. A., et al. (2022) Relationship between the structure and chaperone activity of human αA-Crystallin after its modification with diabetes-associated oxidative agents and protective role of antioxidant compounds, Biochemistry (Moscow), 87, 91-105.
Graw, J. (2009) Genetics of crystallins: Cataract and beyond, Exp. Eye Res., 88, 173-189.
Budnar, B., Tangirala, R., Bakthisaran, R., and Rao, C. M. (2022) Protein aggregation and cataract: Role of age-related modifications and mutations in α-crystallins, Biochemistry (Moscow), in press.
Funding
This work was supported by the Iran National Science Foundation, INSF (Grant No. 99014455).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflicts of interest. This article does not contain description of studies with the involvement of humans or animal subjects performed by the author.
Rights and permissions
About this article
Cite this article
Yousefi, R. Crystallins as Important Pathogenic Targets for Accumulation of Structural Damages Resulting in Protein Aggregation and Cataract Development: Introduction to This Special Issue of Biochemistry (Moscow). Biochemistry Moscow 87, 87–90 (2022). https://doi.org/10.1134/S0006297922020018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922020018