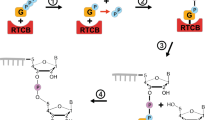
Abstract
Members of the Lsm protein family are found in all three domains of life: bacteria, archaea, and eukarya. They are involved in numerous processes associated with RNA processing and gene expression regulation. A common structural feature of all Lsm family proteins is the presence of the Sm fold consisting of a five-stranded β-sheet and an α-helix at the N-terminus. Heteroheptameric eukaryotic Sm and Lsm proteins participate in the formation of spliceosomes and mRNA decapping. Homohexameric bacterial Lsm protein, Hfq, is involved in the regulation of transcription of different mRNAs by facilitating their interactions with small regulatory RNAs. Furthermore, recently obtained data indicate a new role of Hfq as a ribosome biogenesis factor, as it mediates formation of the productive structure of the 17S rRNA 3′- and 5′-sequences, facilitating their further processing by RNases. Lsm archaeal proteins (SmAPs) form homoheptamers and likely interact with single-stranded uridine-rich RNA elements, although the role of these proteins in archaea is still poorly understood. In this review, we discuss the structural features of the Lsm family proteins from different life domains and their structure–function relationships.






Similar content being viewed by others
Abbreviations
- Sm:
-
Smith antigen
- Lsm protein:
-
Like-Sm (Sm-like) protein
- SmAP:
-
Sm archaeal protein
- Hfq:
-
Qβ host factor, replicase (host factor I participating in the replication of Qβ bacteriophage)
- snRNA:
-
small nuclear RNA
- sRNA:
-
small regulatory RNA
- snRNP:
-
small nuclear ribonucleoprotein
References
Mura, C., Randolph, P. S., Patterson, J., and Cozen, A. E. (2013) Archaeal and eukaryotic homologs of Hfq, RNA Biol., 10, 636-651.
Wilusz, C. J., and Wilusz, J. (2005) Eukaryotic Lsm proteins: lessons from bacteria, Nat. Struct. Mol. Biol., 12, 1031-1036.
Notman, D. D., Kurata, N., and Tan, E. M. (1975) Profiles of antinuclear antibodies in systemic rheumatic diseases, Ann. Intern. Med., 83, 464-469.
Thore, S., Mayer, C., Sauter, C., Weeks, S., and Suck, D. (2003) Crystal structures of the Pyrococcus abyssi Sm core and its complex with RNA: common features of RNA binding in archaea and eukarya, J. Biol. Chem., 278, 1239-1247.
Sun, X. (2002) Predicted structure and phyletic distribution of the RNA-binding protein Hfq, Nucleic Acids Res., 30, 3662-3671.
Møller, T., Franch, T., Højrup, P., Keene, D. R., Bächinger, H. P., et al. (2002) Hfq: a bacterial Sm-like protein that mediates RNA–RNA interaction, Mol. Cell, 9, 23-30.
Murina, V., Lekontseva, N., and Nikulin, A. (2013) Hfq binds ribonucleotides in three different RNA-binding sites, Acta Crystallogr. Sec. D Biol. Crystallogr., 69, 1504-1513.
Wassarman, K. M., Repoila, F., Rosenow, C., Storz, G., and Gottesman, S. (2001) Identification of novel small RNAs using comparative genomics and microarrays, Genes Dev., 15, 1637-1651.
Salgado-Garrido, J., Bragado-Nilsson, E., Kandels-Lewis, S., and Séraphin, B. (1999) Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin, EMBO J., 18, 3451-3462.
Mura, C., Phillips, M., Kozhukhovsky, A., and Eisenberg, D. (2003) Structure and assembly of an augmented Sm-like archaeal protein 14-mer, Proc. Natl. Acad. Sci. USA, 100, 4539-4544.
Collins, B. M., Harrop, S. J., Kornfeld, G. D., Dawes, I. W., Curmi, P. M.., and Mabbutt, B. C. (2001) Crystal structure of a heptameric Sm-like protein complex from archaea: implications for the structure and evolution of snRNPs, J. Mol. Biol., 309, 915-923.
Törö, I., Thore, S., Mayer, C., Basquin, J., Séraphin, B., and Suck, D. (2001) RNA binding in an Sm core domain: X-ray structure and functional analysis of an archaeal Sm protein complex, EMBO J., 20, 2293-22303.
Murina, V. N., and Nikulin, A. D. (2011) RNA-binding Sm-like proteins of bacteria and archaea: similarity and difference in structure and function, Biochemistry (Moscow), 76, 1434-1449, https://doi.org/10.1134/S0006297911130050.
Kambach, C., Walke, S., Young, R., Avis, J. M., De La Fortelle, E., et al. (1999) Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs, Cell, 96, 375-387.
Mura, C., Cascio, D., Sawaya, M. R., and Eisenberg, D. S. (2001) The crystal structure of a heptameric archaeal Sm protein: implications for the eukaryotic snRNP core, Proc. Natl. Acad. Sci. USA, 98, 5532-5537.
Schumacher, M. A., Pearson, R. F., Møller, T., Valentin-Hansen, P., and Brennan, R. G. (2002) Structures of the pleiotropic translational regulator Hfq and an Hfq–RNA complex: a bacterial Sm-like protein, EMBO J., 21, 3546-3556.
Sauter, C., Basquin, J., and Suck, D. (2003) Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli, Nucleic Acids Res., 31, 4091-4098.
Nikulin, A., Stolboushkina, E., Perederina, A., Vassilieva, I., Blaesi, U., et al. (2005) Structure of Pseudomonas aeruginosa Hfq protein, Acta Crystallogr. Sec. D Biol. Crystallogr., 61, 141-146.
Das, D., Kozbial, P., Axelrod, H. L., Miller, M. D., McMullan, D., et al. (2009) Crystal structure of a novel Sm-like protein of putative cyanophage origin at 2.60 Å resolution, Proteins, 75, 296-307.
Törö, I., Basquin, J., Teo-Dreher, H., and Suck, D. (2002) Archaeal Sm proteins form heptameric and hexameric complexes: crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus, J. Mol. Biol., 320, 129-142.
Wu, D., Jiang, S., Bowler, M. W., and Song, H. (2012) Crystal structures of Lsm3, Lsm4 and Lsm5/6/7 from Schizosaccharomyces pombe, PLoS One, 7, e36768.
Naidoo, N., Harrop, S. J., Sobti, M., Haynes, P. A, Szymczyna, B. R., et al. (2008) Crystal structure of Lsm3 octamer from Saccharomyces cerevisiae: implications for Lsm ring organisation and recruitment, J. Mol. Biol., 377, 1357-1371.
Weichenrieder, O. (2014) A trade-off between optimal sequence readout and RNA backbone conformation RNA binding by Hfq and ring-forming (L)Sm proteins, RNA Biol., 11, 537-549.
Weber, G., Trowitzsch, S., Kastner, B., Lührmann, R., and Wahl, M. C. (2010) Functional organization of the Sm core in the crystal structure of human U1 snRNP, EMBO J., 29, 4172-4184.
Wilusz, C. J., and Wilusz, J. (2013) Lsm proteins and Hfq, RNA Biol., 10, 592-601.
Vogel, J., and Luisi, B. F. (2011) Hfq and its constellation of RNA, Nat. Rev. Microbiol., 9, 578-589.
Wagner, E. G. H. (2013) Cycling of RNAs on Hfq, RNA Biol., 10, 619-626.
Tharun, S. (2008) Roles of eukaryotic Lsm proteins in the regulation of mRNA function, Int. Rev. Cell Mol. Biol., 272, 149-189.
Walke, S., Bragado-Nilsson, E., Séraphin, B., and Nagai, K. (2001) Stoichiometry of the Sm proteins in yeast spliceosomal snRNPs supports the heptamer ring model of the core domain, J. Mol. Biol., 308, 49-58.
Licht, K., Medenbach, J., Luhrmann, R., Kambach, C., and Bindereif, A. (2008) 3′-cyclic phosphorylation of U6 snRNA leads to recruitment of recycling factor p110 through LSm proteins, RNA, 14, 1532-1538.
Zaric, B., Chami, M., Rémigy, H., Engel, A., Ballmer-Hofer, K., et al. (2005) Reconstitution, of two recombinant LSm protein complexes reveals aspects of their architecture, assembly, and function, J. Biol. Chem., 280, 16066-16075.
Rinke, J., and Steitz, J. A. (1985) Association of the lupus antigen La with a subset of U6 snRNA molecules, Nucleic Acids Res., 13, 2617-2629.
Shchepachev, V., Wischnewski, H., Missiaglia, E., Soneson, C., and Azzalin, C. M. (2012) Mpn1, mutated in poikiloderma with neutropenia protein 1, is a conserved 3′-to-5′ RNA exonuclease processing U6 small nuclear RNA, Cell Rep., 2, 855-865.
Achsel, T., Stark, H., and Lührmann, R. (2001) The Sm domain is an ancient RNA-binding motif with oligo(U) specificity, Proc. Natl. Acad. Sci. USA, 98, 3685-3689.
Zhou, L., Hang, J., Zhou, Y., Wan, R., Lu, G., et al. (2014) Crystal structures of the Lsm complex bound to the 3′ end sequence of U6 small nuclear RNA, Nature, 506, 116-120.
Tharun, S., He, W., Mayes, A. E., Lennertz, P., Beggs, J. D., and Parker, R. (2000) Yeast Sm-like proteins function in mRNA decapping and decay, Nature, 404, 515-518.
Chen, C.-Y. A., and Shyu, A.-B. (2011) Mechanisms of deadenylation-dependent decay, Wiley Interdiscip. Rev. RNA, 2, 167-183.
Chowdhury, A., Mukhopadhyay, J., and Tharun, S. (2007) The decapping activator Lsm1p-7p-Pat1p complex has the intrinsic ability to distinguish between oligoadenylated and polyadenylated RNAs, RNA, 13, 998-1016.
Braun, J. E., Tritschler, F., Haas, G., Igreja, C., Truffault, V., et al. (2010) The C-terminal α-α superhelix of Pat is required for mRNA decapping in metazoa, EMBO J., 29, 2368-2380.
Zhou, L., Zhou, Y., Hang, J., Wan, R., Lu, G., et al. (2014) Crystal structure and biochemical analysis of the heptameric Lsm1-7 complex, Cell Res., 24, 497-500.
Wu, D., Muhlrad, D., Bowler, M. W., Jiang, S., Liu, Z., et al. (2014) Lsm2 and Lsm3 bridge the interaction of the Lsm1-7 complex with Pat1 for decapping activation, Cell Res., 24, 233-246.
Rissland, O. S., and Norbury, C. J. (2009) Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover, Nat. Struct. Mol. Biol., 16, 616-623.
Morozov, I. Y., Jones, M. G., Razak, A. A., Rigden, D. J., and Caddick, M. X. (2010) CUCU modification of mRNA promotes decapping and transcript degradation in Aspergillus nidulans, Mol. Cel. Biol., 30, 460-469.
Morozov, I. Y., Jones, M. G., Gould, P. D., Crome, V., Wilson, J. B., et al. (2012) mRNA 3′-tagging is induced by nonsense-mediated decay and promotes ribosome dissociation, Mol. Cel. Biol., 32, 2585-2595.
Franze de Fernandez, M. T., Hayward, W. S., and August, J. T. (1972) Bacterial proteins required for replication of phage Q ribonucleic acid. Purification and properties of host factor I, a ribonucleic acid-binding protein, J. Biol. Chem., 247, 824-831.
Sauer, E. (2013) Structure and RNA-binding properties of the bacterial LSm protein Hfq, RNA Biol., 10, 610-618.
Updegrove, T. B., Zhang, A., and Storz, G. (2016) Hfq: the flexible RNA matchmaker, Curr. Opin. Microbiol., 30, 133-138.
Otaka, H., Ishikawa, H., Morita, T., and Aiba, H. (2011) PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action, Proc. Natl. Acad. Sci. USA, 108, 13059-13064.
Wang, W., Wang, L., Wu, J., Gong, Q., and Shi, Y. (2013) Hfq-bridged ternary complex is important for translation activation of rpoS by DsrA, Nucleic Acids Res., 41, 5938-5948.
Sauer, E., and Weichenrieder, O. (2011) Structural basis for RNA 3′-end recognition by Hfq, Proc. Natl. Acad. Sci. USA, 108, 13065-13070.
Zhang, A., Schu, D. J., Tjaden, B. C., Storz, G., and Gottesman, S. (2013) Mutations in interaction surfaces differentially impact E. coli Hfq association with small RNAs and their mRNA targets, J. Mol. Biol., 425, 3678-3697.
Panja, S., Santiago-Frangos, A., Schu, D. J., Gottesman, S., and Woodson, S. A. (2015) Acidic residues in the Hfq chaperone increase the selectivity of sRNA binding and annealing, J. Mol. Biol., 427, 3491-3500.
Link, T. M., Valentin-Hansen, P., and Brennan, R. G. (2009) Structure of Escherichia coli Hfq bound to polyriboadenylate RNA, Proc. Natl. Acad. Sci. USA, 106, 19292-19297.
Someya, T., Baba, S., Fujimoto, M., Kawai, G., Kumasaka, T., and Nakamura, K. (2012) Crystal structure of Hfq from Bacillus subtilis in complex with SELEX-derived RNA aptamer: insight into RNA-binding properties of bacterial Hfq, Nucleic Acids Res., 40, 1856-1867.
Schu, D. J., Zhang, A., Gottesman, S., and Storz, G. (2015) Alternative Hfq-sRNA interaction modes dictate alternative mRNA recognition, EMBO J., 34, 2557-2573.
Sauer, E., Schmidt, S., and Weichenrieder, O. (2012) Small RNA binding to the lateral surface of Hfq hexamers and structural rearrangements upon mRNA target recognition, Proc. Natl. Acad. Sci. USA, 109, 9396-9401.
Sobrero, P., and Valverde, C. (2012) The bacterial protein Hfq: much more than a mere RNA-binding factor, Crit. Rev. Microbiol., 38, 276-299.
Dimastrogiovanni, D., Fröhlich, K. S., Bandyra, K. J., Bruce, H. A., Hohensee, S., et al. (2014) Recognition of the small regulatory RNA RydC by the bacterial Hfq protein, ELife, 3, e05375.
Robinson, K. E., Orans, J., Kovach, A. R., Link, T. M., and Brennan, R. G. (2014) Mapping Hfq–RNA interaction surfaces using tryptophan fluorescence quenching, Nucleic Acids Res., 42, 2736-2749.
Vincent, H. A., Henderson, C. A., Ragan, T. J., Garza-Garcia, A., Cary, P. D., et al. (2012) Characterization of Vibrio cholerae Hfq provides novel insights into the role of the Hfq C-terminal region, J. Mol. Biol., 420, 56-69.
Lee, T., and Feig, A. L. (2008) The RNA binding protein Hfq interacts specifically with tRNAs, RNA, 14, 514-523.
Zhang, A., Wassarman, K. M., Rosenow, C., Tjaden, B. C., Storz, G., and Gottesman, S. (2003) Global analysis of small RNA and mRNA targets of Hfq, Mol. Microbiol., 50, 1111-1124.
Azam, T. A., Hiraga, S., and Ishihama, A. (2000) Two types of localization of the DNA-binding proteins within the Escherichia coli nucleoid, Genes Cells, 5, 613-626.
Malabirade, A., Jiang, K., Kubiak, K., Diaz-Mendoza, A., Liu, F., et al. (2017) Compaction and condensation of DNA mediated by the C-terminal domain of Hfq, Nucleic Acids Res., 45, 7299-7308.
Andrade, J. M., Pobre, V., Matos, A. M., and Arraiano, C. M. (2012) The crucial role of PNPase in the degradation of small RNAs that are not associated with Hfq, RNA, 18, 844-855.
Moll, I., Leitsch, D., Steinhauser, T., and Bläsi, U. (2003) RNA chaperone activity of the Sm-like Hfq protein, EMBO Rep., 4, 284-289.
Régnier, P., and Hajnsdorf, E. (2013) The interplay of Hfq, poly(A) polymerase I and exoribonucleases at the 3′ ends of RNAs resulting from Rho-independent termination: a tentative model, RNA Biol., 10, 602-609.
Sukhodolets, M. V., and Garges, S. (2003) Interaction of Escherichia coli RNA polymerase with the ribosomal protein S1 and the Sm-like ATPase Hfq, Biochemistry, 42, 8022-8034.
Rabhi, M., Espéli, O., Schwartz, A., Cayrol, B., Rahmouni, A. R., et al. (2011) The Sm-like RNA chaperone Hfq mediates transcription antitermination at Rho-dependent terminators, EMBO J., 30, 2805-2816.
Dos Santos, R. F., Arraiano, C. M., and Andrade, J. M. (2019) New molecular interactions broaden the functions of the RNA chaperone Hfq, Curr. Genet., 65, 1313-1319.
Andrade, J. M., Santos, R. F., Chelysheva, I., Ignatova, Z., and Arraiano, C. M. (2018) The RNA-binding protein Hfq is important for ribosome biogenesis and affects translation fidelity, EMBO J., 37, 1-13.
Strader, M. B., Hervey, W. J., Costantino, N., Fujigaki, S., Chen, C. Y., et al. (2013) A coordinated proteomic approach for identifying proteins that interact with the E. coli ribosomal protein S12, J. Proteome Res., 12, 1289-1299.
Yusupov, M. M., Yusupova, G. Z., Baucom, A., Lieberman, K., Earnest, T. N., et al. (2001) Crystal structure of the ribosome at 5.5 Å resolution, Science, 292, 883-896.
Cukras, A. R., Southworth, D. R., Brunelle, J. L., Culver, G. M., and Green, R. (2003) Ribosomal proteins S12 and S13 function as control elements for translocation of the mRNA:tRNA complex, Mol. Cell, 12, 321-328.
Nielsen, J. S., Boggild, A., Andersen, C. B. F., Nielsen, G., Boysen, A., et al. (2007) An Hfq-like protein in archaea: crystal structure and functional characterization of the Sm protein from Methanococcus jannaschii, RNA, 13, 2213-2223.
Nikulin, A., Mikhailina, A., Lekontseva, N., Balobanov, V., Nikonova, E., and Tishchenko, S. (2017) Characterization of RNA-binding properties of the archaeal Hfq-like protein from Methanococcus jannaschii, J. Biomol. Struct. Dyn., 35, 1615-1628.
Märtens, B., Hou, L., Amman, F., Wolfinger, M. T., Evguenieva-Hackenberg, E., and Bläsi, U. (2017) The SmAP1/2 proteins of the crenarchaeon Sulfolobus solfataricus interact with the exosome and stimulate A-rich tailing of transcripts, Nucleic Acids Res., 45, 7938-7949.
Märtens, B., Bezerra, G. A., Kreuter, M. J., Grishkovskaya, I., Manica, A., Arkhipova, V., et al. (2015) The heptameric SmAP1 and SmAP2 proteins of the crenarchaeon Sulfolobus Solfataricus bind to common and distinct RNA targets, Life (Basel), 5, 1264-1281.
Fischer, S., Benz, J., Späth, B., Maier, L.-K., Straub, J., et al. (2010) The archaeal Lsm protein binds to small RNAs, J. Biol. Chem., 285, 34429-34438.
Lekontseva, N., Mikhailina, A., Fando, M., Kravchenko, O., Balobanov, V., et al. (2020) Crystal structures and RNA-binding properties of Lsm proteins from archaea Sulfolobus acidocaldarius and Methanococcus vannielii: similarity and difference of the U-binding mode, Biochimie, 175, 1-12.
Funding
This work was supported by the Russian Science Foundation (project no. 19-74-20186).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lekontseva, N.V., Stolboushkina, E.A. & Nikulin, A.D. Diversity of LSM Family Proteins: Similarities and Differences. Biochemistry Moscow 86 (Suppl 1), S38–S49 (2021). https://doi.org/10.1134/S0006297921140042
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921140042