Abstract
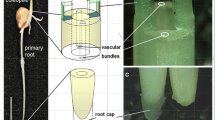
According to the proposed theory, the starch-rich particles (statoliths) help the plant to convert the signals from Earth’s motions into the signals necessary for the plant to perceive its orientation relative to the gravity vector while moving freely because of inertia in the sensory cells (statocytes) of roots and stems. Motions of the Earth are never constant, which, in particular, refers to the so-called polar motions and oscillations of the planet’s rotation axis. Statoliths at any given moment move in the cytoplasmic liquid of statocytes due to inertial motion initiated by the action of the Earth’s movements, maintaining the trajectory set by the previous movement of the oscillating planet. Unlike statoliths, the walls of a statocyte move in space along with the entire plant and with the Earth, in strict accordance with the current direction of motion of the planet’s axis. This leads to the inevitable collision of statoliths with the statocytic wall/membrane. Cytoplasmic liquid, as a substance that is not able to maintain its shape, does not interfere with the inertial motions of the statoliths and collision with the wall of the statocyte. By striking the membrane, statoliths cause the release of ions and other factors at the impact site, which further participate in the gravitropic process. Pressure of the sediment of statoliths at the bottom of the statocyte, as well as position of this sediment, are not the defining factors of gravitropism.
Similar content being viewed by others
References
Darwin, C., and Darwin, F (1880) The Power of Movement in Plants, John Murray, London.
Haberlandt, G. (1914) Physiological plant anatomy, Macmillan and Company.
Sack, F. D. (1991) Plant gravity sensing, Int. Rev. Cytol., 127, 193-252.
Kiss, J. Z. (2000) Mechanisms of the early phases of plant gravitropism, Crit. Rev. Plant Sci., 19, 551-573, https://doi.org/10.1080/07352680091139295.
Nakamura, M., Nishimura, T., and Morita, M. T. (2019) Gravity sensing and signal conversion in plant gravitropism, J. Exp. Botany, 70, 3495-3506, https://doi.org/10.1093/jxb/erz158.
Abe, Y., Meguriya, K., Matsuzaki, T., Sugiyama, T., Yoshikawa, H. Y., et al. (2020) Micromanipulation of amyloplasts with optical tweezers in Arabidopsis stems, Plant Biotechnol., 37, 405-415, https://doi.org/10.5511/plantbiotechnology.20.1201a.
Fukaki, H., Wysocka-Diller, J., Kato, T., Fujisawa, H., Benfey, P. N., and Tasaka, M. (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana, Plant J., 14, 425-430.
Takahashi, K., Takahashi, H., Furuichi, T., Toyota, M., Furutani-Seiki, M., et al. (2021) Gravity sensing in plant and animal cells, NPJ Microgravity, 7, 2, https://doi.org/10.1038/s41526-020-00130-8.
Muthert, L., Izzo, L. G., van Zanten, M., and Aronne, G. (2020) Root tropisms: investigations on earth and in space to unravel plant growth direction, Front. Plant Sci., 10, 1807, https://doi.org/10.3389/fpls.2019.01807.
Morita, M. T., and Tasaka, M. (2004) Gravity sensing and signaling, Curr. Opin. Plant Biol., 7, 712-718, https://doi.org/10.1016/j.pbi.2004.09.001.
Staves M. P. (1997) Cytoplasmic streaming and gravity sensing in Chara internodal cells, Planta, 203 (Suppl 1), S79-S84, https://doi.org/10.1007/pl00008119.
Perbal, G., and Perbal, P. (1976) Geoperception in the lentil root cap, Physiol. Plant., 37, 42-48.
Kondrachuk, A. V. (2001) Theoretical considerations of plant gravisensing, Adv. Space Res., 27, 907-914, https://doi.org/10.1016/s0273-1177(01)00187-9.
Tatsumi, H., Furuichi, T., Nakano, M., Toyota, M., Hayakawa, K., et al. (2014) Mechanosensitive channels are activated by stress in the actin stress fibres, and could be involved in gravity sensing in plants, Plant Biol. J., 16, 18-22, https://doi.org/10.1111/plb.12095.
Guharay, F., and Sachs, F. (1984) Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle, J. Physiol., 352, 685.
Levernier, N., Pouliquen, O., and Forterre, Y. (2021) An integrative model of plant gravitropism linking statoliths position and auxin transport, Front. Plant Sci., 12, 651928, https://doi.org/10.3389/fpls.2021.651928.
Chauvet, H., Pouliquen, O., Forterre, Y., Legué, V., and Moulia, B. (2016) Inclination not force is sensed by plants during shoot gravitropism, Sci. Rep., 6, 35431, https://doi.org/10.1038/srep35431.
Pouliquen, O., Forterre, Y., Bérut, A., Chauvet, H., Bizet, F., et al. (2017) A new scenario for gravity detection in plants: the position sensor hypothesis, Phys. Biol., 14, 035005, https://doi.org/10.1088/1478-3975/aa6876.
Bérut, A., Chauvet, H., Legué, V., Moulia, B., Pouliquen, O., and Forterre, Y. (2018) Gravisensors in plant cells behave like an active granular liquid, Proc. Natl. Acad. Sci. USA, 115, 5123, https://doi.org/10.1073/pnas.1801895115.
Perbal, G., and Driss-Ecole, D. (2003) Mechanotransduction in gravisensing cells, Trends Plant Sci., 8, 498-504, https://doi.org/10.1016/j.tplants.2003.09.005.
Richter, P., Strauch, S. M., and Lebert, M. (2019) Disproval of the starch-amyloplast hypothesis? Trends Plant Sci., 24, 291-293, https://doi.org/10.1016/j.tplants.2019.02.008.
Häder, D. P., Braun, M., Grimm, D., and Hemmersbach, R. (2017) Gravireceptors in eukaryotes-a comparison of case studies on the cellular level, NPJ Microgravity, 3, 13, https://doi.org/10.1038/s41526-017-0018-8.
Limbach, C., Hauslage, J., Schäfer, C, and Braun, M. (2005) How to activate a plant gravireceptor. Early mechanisms of gravity sensing studied in characean rhizoids during parabolic flights, Plant Physiol., 139, 1030-1040, https://doi.org/10.1104/pp.105.068106.
Meroz, Y., and Bastien, R. (2014) Stochastic processes in gravitropism, Front. Plant Sci., 5, 674, https://doi.org/10.3389/fpls.2014.00674.
Morita, M. T. (2010) Directional gravity sensing in gravitropism, Annu. Rev. Plant Biol., 61, 705-720, https://doi.org/10.1146/annurev.arplant.043008.092042.
Caspar, T., and Pickard, B. G. (1989) Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing, Planta, 177, 185-197.
Dehant, V., Laguerre, R., Rekier, J., Rivoldini, A., Triana, S. A., et al. (2017) Understanding the effects of the core on the nutation of the Earth, Geodesy Geodynamics, 8, 389-395.
Zajdel, R., Sośnica, K., Bury, G., Dach, R., Prange, L., and Kazmierski, K. (2021) Sub-daily polar motion from GPS, GLONASS, and Galileo, J. Geodesy, 95, 1-27.
Dehant, V., Triana, S. A., Rekier, J., Trinh, A., Zhu, P., et al. (2020) Progress in understanding nutations, Astrometry, Earth Rotation, and Reference Systems in the GAIA era, 233-236, URL: https://dial.uclouvain.be/pr/boreal/object/boreal%3A249469/datastream/PDF_01/view.
Dehant, V., and Mathews, P. M. (2015) Precession, Nutation, and Wobble of the Earth, Cambridge University Press, 536 p.
Chao, B. F. (2017) Dynamics of the inner core wobble under mantle-inner core gravitational interactions, J. Geophys. Res. Solid Earth, 122, 7437-7448, https://doi.org/10.1002/2017JB014405.
Zhu, P., Triana, S. A., Rekier, J., Trinh, A., and Dehant, V. (2021) Quantification of corrections for the main lunisolar nutation components and analysis of the free core nutation from VLBI-observed nutation residuals, J. Geodesy, 95, 1-15.
Konstantinova, N., Korbei, B., and Luschnig, C. (2021) Auxin and root gravitropism: addressing basic cellular processes by exploiting a defined growth response, Int. J. Mol. Sci., 22, 2749, https://doi.org/10.3390/ijms22052749.
Jiao, Z., Du, H., Chen, S., Huang, W., and Ge, L. (2021) LAZY gene family in plant gravitropism, Front. Plant Sci., 11, 606241, https://doi.org/10.3389/fpls.2020.606241.
Sack, F. D., Suyemoto, M. M., and Leopold, A. C. (1986) Amyloplast sedimentation and organelle saltation in living corn columella cells, Am. J. Bot., 73, 1692-1698.
Nakamura, M., Toyota, M., Tasaka, M., and Morita, M. T. (2015) Live cell imaging of cytoskeletal and organelle dynamics in gravity-sensing cells in plant gravitropism, Methods Mol. Biol., 1309, 57-69, https://doi.org/10.1007/978-1-4939-2697-8_6.
Zhang, Y., Xiao, G., Wang, X., Zhang, X., and Friml, J. (2019) Evolution of fast root gravitropism in seed plants, Nat. Commun., 10, 3480, https://doi.org/10.1038/s41467-019-11471-8.
Toyota, M., Furuichi, T., Sokabe, M., and Tatsumi, H. (2013) Analyses of a gravistimulation-specific Ca2+ signature in Arabidopsis using parabolic flights, Plant Physiol., 163, 543-554, https://doi.org/10.1104/pp.113.223313.
Nakano, M., Furuichi, T., Sokabe, M., Iida, H., and Tatsumi, H. (2021) The gravistimulation-induced very slow Ca2+ increase in Arabidopsis seedlings requires MCA1, a Ca2+-permeable mechanosensitive channel, Sci. Rep., 11, 227, https://doi.org/10.1038/s41598-020-80733-z.
Oikawa, T., Ishimaru, Y., Munemasa, S., Takeuchi, Y., Washiyama, K., et al. (2018) Ion channels regulate nyctinastic leaf opening in Samanea saman, Curr. Biol., 28, 2230-2238.e7, https://doi.org/10.1016/j.cub.2018.05.042.
Hasenstein, K.H. (2009) Plant responses to gravity – insights and extrapolations from ground studies, Gravitational Space Biol., 22, 21, https://go.gale.com/ps/i.do?p=AONE&u=anon~babadf1b&id=GALE|A348311756&v=2.1&it=r&sid=googleScholar&asid=b061b220.
Wolfe, J., and Steponkus, P. L. (1981) The stress-strain relation of the plasma membrane of isolated plant protoplasts, Biochim. Biophys. Acta, 643, 663-668.
Sachs, J. (1887) Lectures on the Physiology of Plants, Clarendon Press, Oxford, https://doi.org/10.5962/bhl.title.54852.
Dumais, J. (2013) Beyond the sine law of plant gravitropism, Proc. Natl. Acad. Sci. USA, 110, 391-392.
Iino, M., Tarui, Y., and Uematsu, C. (1996) Gravitropism of maize and rice coleoptiles: dependence on the stimulation angle, Plant Cell Environ., 19, 1160-1168.
Toyota, M., Furuichi, T., Tatsumi, H., and Sokabe, M. (2008) Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings, Plant Physiol., 146, 505-514, https://doi.org/10.1104/pp.107.106450.
Digby, J., and Firn, R. D. (1995) The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture, Plant Cell Environ., 18, 1434-1440, https://doi.org/10.1111/j.1365-3040.1995.tb00205.x.
Saito, C., Morita, M. T., Kato, T., and Tasaka, M. (2005) Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the arabidopsis inflorescence stem, Plant Cell, 17, 548-558, https://doi.org/10.1105/tpc.104.026138.
Nakamura, M., Toyota, M., Tasaka, M., and Morita, M. T. (2011) An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and F-actin in gravity sensing, Plant Cell, 23, 1830-1848.
Zou, J. J., Zheng, Z. Y., Xue, S., Li, H. H., Wang, Y. R., and Le, J. (2016) The role of Arabidopsis Actin-Related Protein 3 in amyloplast sedimentation and polar auxin transport in root gravitropism, J. Exp. Botany, 67, 5325-5337, https://doi.org/10.1093/jxb/erw294.
Baluska, F., Kreibaum, A., Vitha, S., Parker, J. S., Barlow, P. W., and Sievers, A. (1997) Central root cap cells are depleted of endoplasmic microtubules and actin microfilament bundles: implications for their role as gravity-sensing statocytes, Protoplasma, 196, 212-223, https://doi.org/10.1007/BF01279569.
Knight, T. A. (1806) On the direction of the radicle and germen during the vegetation of seeds, Philos. Trans. R. Soc. Lond., 96, 108-120.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflicts of interest in financial or any other sphere. This article does not contain any studies with human participants or animals performed by the author.
Rights and permissions
About this article
Cite this article
Olovnikov, A.M. Role of the Earth’s Motions in Plant Orientation – Planetary Mechanism. Biochemistry Moscow 86, 1388–1394 (2021). https://doi.org/10.1134/S0006297921110031
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921110031