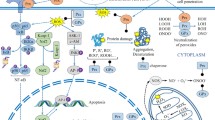
Abstract
The review presents current concepts of the molecular mechanisms of oxidative stress development and describes main stages of the free-radical reactions in oxidative stress. Endogenous and exogenous factors of the oxidative stress development, including dysfunction of cell oxidoreductase systems, as well as the effects of various external physicochemical factors, are discussed. The review also describes the main components of the antioxidant defense system and stages of its evolution, with a special focus on peroxiredoxins, glutathione peroxidases, and glutathione S-transferases, which share some phylogenetic, structural, and catalytic properties. The substrate specificity, as well as the similarities and differences in the catalytic mechanisms of these enzymes, are discussed in detail. The role of peroxiredoxins, glutathione peroxidases, and glutathione S-transferases in the regulation of hydroperoxide-mediated intracellular and intercellular signaling and interactions of these enzymes with receptors and non-receptor proteins are described. An important contribution of hydroperoxide-reducing enzymes to the antioxidant protection and regulation of such cell processes as growth, differentiation, and apoptosis is demonstrated.


Similar content being viewed by others
Abbreviations
- CP :
-
peroxidatic cysteine
- CR :
-
resolving cysteine
- Grx:
-
glutaredoxin
- GPx:
-
glutathione peroxidase
- GR:
-
glutathione reductase
- GSH:
-
glutathione, reduced
- HO• :
-
hydroxyl radical
- In• :
-
antioxidant free radical
- L• :
-
lipid alkyl radical
- LH:
-
polyunsaturated fatty acid
- LO• :
-
lipoxal radical
- LOO• :
-
lipoperoxy radical
- LOOH:
-
lipid hydroperoxide
- RHS:
-
reactive halogen species
- ROS:
-
reactive oxygen species
- RNS:
-
reactive nitrogen species
- LPO:
-
lipid peroxidation
- O2 •− :
-
superoxide anion radical
- GST:
-
glutathione S-transferase
- PDI:
-
protein disulfide isomerase
- Prx:
-
peroxiredoxin
- RO• :
-
alkoxy radical
- SOD:
-
superoxide dismutase
- Trx:
-
thioredoxin
- TrxR:
-
thioredoxin reductase
References
Menshchikova, E. B., Lankin, V. Z., Zenkov, N. K., Bondar, I. A., Krugovykh, N. F., and Trufakin, V. A. (2006) Oxidative Stress. Peroxidants and Antioxidants, Slovo, Moscow.
Hernansanz-Agustín, P., and Enríquez, J. A. (2021) Generation of reactive oxygen species by mitochondria, Antioxidants (Basel), 10, 415, https://doi.org/10.3390/antiox10030415.
Chernyak, B. V., Izyumov, D. S., Lyamzaev, K. G., Pashkovskaya, A. A., Pletjushkina, O. Y., et al. (2006) Production of reactive oxygen species in mitochondria of HeLa cells under oxidative stress, Biochim. Biophys. Acta Bioenerg., 1757, 525-534, https://doi.org/10.1016/j.bbabio.2006.02.019.
McCord, J. M. (2000) The evolution of free radicals and oxidative stress, Am. J. Med., 108, 652-659.
Brand, M. D. (2016) Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling, Free Radic. Biol. Med., 100, 14-31, https://doi.org/10.1016/j.freeradbiomed.2016.04.001.
Crapo, J. D., and Tierney, D. F. (1974) Superoxide dismutase and pulmonary oxygen toxicity, Am. J. Physiol., 226, 1401-1407.
Di Meo, S., Reed, T. T., Venditti, P., and Victor, V. M. (2016) Role of ROS and RNS sources in physiological and pathological conditions, Oxid. Med. Cell. Longev., 2016, 1245049, https://doi.org/10.1155/2016/1245049.
Halliwell, B. (2020) Reflections of an aging free radical, Free Radic. Biol. Med., 161, 234-245, https://doi.org/10.1016/j.freeradbiomed.2020.10.010.
Vladimirov, Y. A., and Proskurnina, E. V. (2009) Free radicals and cell chemiluminescence, Biochemistry (Moscow), 74, 1545-1566, https://doi.org/10.1134/S0006297909130082.
Bruskov, V. I., Karp, O. E., Garmash, S. A., Shtarkman, I. N., Chernikov, A. V., and Gudkov, S. V. (2012) Prolongation of oxidative stress by long-lived reactive protein species induced by X-ray radiation and their genotoxic action, Free Radic. Res., 46, 1280-1290, https://doi.org/10.3109/10715762.2012.709316.
Soodaeva, S. K., Klimanov, I. A., and Nikitina, L. Y. (2017) Nitrosative and oxidative stresses in respiratory diseases, Pulmonologiya, 27, 262-273, https://doi.org/10.18093/0869-0189-2017-27-2-262-273.
Schöneich, C. (2019) Thiyl radical reactions in the chemical degradation of pharmaceutical proteins, Molecules, 24, 4357, https://doi.org/10.3390/molecules24234357.
Panov, A. (2018) Perhydroxyl radical (HO2^(•)) as inducer of the isoprostane lipid peroxidation in mitochondria, Mol. Biol. (Mosk)., 52, 347-359, https://doi.org/10.7868/S0026898418030011.
Osipov, A. N., Borisenko, G. G., and Vladimirov, Y. A. (2007) Biological activity of hemoprotein nitrosyl complexes, Biochemistry (Moscow), 72, 1491-1504, https://doi.org/10.1134/S0006297907130068.
Collin, F. (2019) Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases, Int. J. Mol. Sci., 20, 2407, https://doi.org/10.3390/ijms20102407.
Dutton, A. S., Fukuto, J. M., and Houk, K. N. (2005) Theoretical reduction potentials for nitrogen oxides from CBS-QB3 energetics and (C)PCM solvation calculations, Inorg. Chem., 44, 4024-4028, https://doi.org/10.1021/ic048734q.
Berg, J., Tymoczko, J., and Stryer, L. (2002) Biochemistry. 5th Edn., N. Y., W H Freeman.
Panasenko, O. M., Torkhovskaya, T. I., Gorudko, I. V., and Sokolov, A. V. (2020) The Role of halogenative stress in atherogenic modification of low-density lipoproteins, Biochemistry (Moscow), 85 (Suppl 1), 34-55, https://doi.org/10.1134/S0006297920140035.
Di Mascio, P., Martinez, G. R., Miyamoto, S., Ronsein, G. E., Medeiros, M. H. G., and Cadet, J. (2019) Singlet molecular oxygen reactions with nucleic acids, lipids, and proteins, Chem. Rev., 119, 2043-2086, https://doi.org/10.1021/acs.chemrev.8b00554.
Sies, H., and Jones, D. P. (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents, Nat. Rev. Mol. Cell Biol., 21, 363-383, https://doi.org/10.1038/s41580-020-0230-3.
Kohlgrüber, S., Upadhye, A., Dyballa-Rukes, N., McNamara, C. A., and Altschmied, J. (2017) Regulation of transcription factors by reactive oxygen species and nitric oxide in vascular physiology and pathology, Antioxid. Redox Signal., 26, 679-699, https://doi.org/10.1089/ars.2016.6946.
Forrester, S. J., Kikuchi, D. S., Hernandes, M. S., Xu, Q., and Griendling, K. K. (2018) Reactive oxygen species in metabolic and inflammatory signaling, Circ. Res., 122, 877-902, https://doi.org/10.1161/circresaha.117.311401.
Hoffmann, M. H., and Griffiths, H. R. (2018) The dual role of reactive oxygen species in autoimmune and inflammatory diseases: evidence from preclinical models, Free Radic. Biol. Med., 125, 62-71, https://doi.org/10.1016/j.freeradbiomed.2018.03.016.
Cadet, J., and Davies, K. J. A. (2017) Oxidative DNA damage & repair: an introduction, Free Radic. Biol. Med., 107, 2-12, https://doi.org/10.1016/j.freeradbiomed.2017.03.030.
Chernikov, A. V., Gudkov, S. V., Usacheva, A. M., and Bruskov, V. I. (2017) Exogenous 8-oxo-7,8-dihydro-2′-deoxyguanosine: biomedical properties, mechanisms of action, and therapeutic potential, Biochemistry (Moscow), 82, 1686-1701, https://doi.org/10.1134/S0006297917130089.
Chao, M. R., Evans, M. D., Hu, C. W., Ji, Y., Møller, P., et al. (2021) Biomarkers of nucleic acid oxidation – a summary state-of-the-art, Redox Biol., 42, 101872, https://doi.org/10.1016/j.redox.2021.101872.
Poetsch, A. R. (2020) The genomics of oxidative DNA damage, repair, and resulting mutagenesis, Comput. Struct. Biotechnol. J., 18, 207-219, https://doi.org/10.1016/j.csbj.2019.12.013.
Davies, M. J. (2016) Protein oxidation and peroxidation, Biochem. J., 473, 805-825, https://doi.org/10.1042/BJ20151227.
Kehm, R., Baldensperger, T., Raupbach, J., and Höhn, A. (2021) Protein oxidation – formation mechanisms, detection and relevance as biomarkers in human diseases, Redox Biol., 42, 101901, https://doi.org/10.1016/j.redox.2021.101901.
Lankin, V. Z., Shumaev, K. B., Tikhaze, A. K., and Kurganov, B. I. (2017) Influence of dicarbonyls on kinetic characteristics of glutathione peroxidase, Dokl. Biochem. Biophys., 475, 287-290, https://doi.org/10.1134/S1607672917040123.
Lankin, V. Z., Tikhaze, A. K., Konovalova, G. G., and Kozachenko, A. I. (1999) Concentration-dependent inversion of antioxidant and prooxidant effects of β-carotene in tissues in vivo, Bull. Exp. Biol. Med., 128, 930-932, https://doi.org/10.1007/bf02438088.
Braakman, R. (2019) Evolution of cellular metabolism and the rise of a globally productive biosphere, Free Radic. Biol. Med., 140, 172-187, https://doi.org/10.1016/j.freeradbiomed.2019.05.004.
Ittarat, W., Sato, T., Kitashima, M., Sakurai, H., Inoue, K., and Seo, D. (2021) Rubredoxin from the green sulfur bacterium Chlorobaculum tepidum donates a redox equivalent to the flavodiiron protein in an NAD(P)H dependent manner via ferredoxin-NAD(P)+ oxidoreductase, Arch. Microbiol., 203, 799-808, https://doi.org/10.1007/s00203-020-02079-4.
Ding, H., and Demple, B. (2000) Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator, Proc. Natl. Acad. Sci. USA, 97, 5146-5150, https://doi.org/10.1073/pnas.97.10.5146.
Case, A. J. (2017) On the origin of superoxide dismutase: an evolutionary perspective of superoxide-mediated redox signaling, Antioxidants (Basel), 6, 82, https://doi.org/10.3390/antiox6040082.
Zorov, D. B., Andrianova, N. V., Babenko, V. A., Bakeeva, L. E., Zorov, S. D., et al. (2020) Nonphosphorylating oxidation in mitochondria and related processes, Biochemistry (Moscow), 85, 1570-1577, https://doi.org/10.1134/S0006297920120093.
Lankin, V. Z., Vandyshev, D. B., Tikhaze, A. K., Kosykh, V. A., and Pomoǐnetskiǐ, V. D. (1981) Effect of hyperoxia on superoxide dismutase and glutathione lipoperoxidase activity in mouse tissues, Dokl. Akad. Nauk SSSR, 259, 229-231.
Schröder, E., and Eaton, P. (2008) Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations, Curr. Opin. Pharmacol., 8, 153-159, https://doi.org/10.1016/j.coph.2007.12.012.
Rhee, S. G., Woo, H. A., and Kang, D. (2018) The role of peroxiredoxins in the transduction of H2O2 Signals, Antioxidants Redox Signal., 28, 537-557, https://doi.org/10.1089/ars.2017.7167.
Olson, K. R. (2020) Reactive oxygen species or reactive sulfur species: why we should consider the latter, J. Exp. Biol., 223, jeb196352, https://doi.org/10.1242/jeb.196352.
Lu, J., and Holmgren, A. (2014) The thioredoxin antioxidant system, Free Radic. Biol. Med., 66, 75-87, https://doi.org/10.1016/j.freeradbiomed.2013.07.036.
Balsera, M., and Buchanan, B. B. (2019) Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress, Free Radic. Biol. Med., 140, 28-35, https://doi.org/10.1016/j.freeradbiomed.
Ingles-Prieto, A., Ibarra-Molero, B., Delgado-Delgado, A., Perez-Jimenez, R., Fernandez, J. M., et al. (2013) Conservation of protein structure over four billion years, Structure, 21, 1690-1697, https://doi.org/10.1016/j.str.2013.06.020.
Johansson, L., Gafvelin, G., and Arnér, E. S. J. (2005) Selenocysteine in proteins – properties and biotechnological use, Biochim. Biophys. Acta Gen. Subj., 1726, 1-13, https://doi.org/10.1016/j.bbagen.2005.05.010.
Atkinson, H. J., and Babbitt, P. C. (2009) Glutathione transferases are structural and functional outliers in the thioredoxin fold, Biochemistry, 48, 11108-11116, https://doi.org/10.1021/bi901180v.
Pan, J. L., and Bardwell, J. C. A. (2006) The origami of thioredoxin-like folds, Protein Sci., 15, 2217-2227, https://doi.org/10.1110/ps.062268106.
Modi, T., Huihui, J., Ghosh, K., and Ozkan, S. B. (2018) Ancient thioredoxins evolved to modern day stability-function requirement by altering native state ensemble, Philos. Trans. R Soc. Lond. B Biol. Sci., 373, 20170184, https://doi.org/10.1098/rstb.2017.0184.
Matsui, M., Oshima, M., Oshima, H., Takaku, K., Maruyama, T. (1996) Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene, Dev. Biol., 178, 179-185, https://doi.org/10.1006/dbio.1996.0208.
Nonn, L., Williams, R. R., Erickson, R. P., and Powis, G. (2003) The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice, Mol. Cell. Biol., 23, 916-922, https://doi.org/10.1128/MCB.23.3.916-922.2003.
Mitchell, D. A., Morton, S. U., Fernhoff, N. B., and Marletta, M. A. (2007) Thioredoxin is required for S-nitrosation of procaspase-3 and the inhibition of apoptosis in Jurkat cells, Proc. Natl. Acad. Sci. USA, 104, 11609-11614, https://doi.org/10.1073/pnas.0704898104.
Qayyum, N., Haseeb, M., Kim, M. S., Choi, S. (2021) Role of thioredoxin-interacting protein in diseases and its therapeutic outlook, Int. J. Mol. Sci., 22, 2754, https://doi.org/10.3390/ijms22052754.
Benhar, M., Shytaj, I. L., Stamler, J. S., and Savarino, A. (2016) Dual targeting of the thioredoxin and glutathione systems in cancer and HIV, J. Clin. Invest., 126, 1630-1639, https://doi.org/10.1172/JCI85339.
Lee, S., Kim, S. M., and Lee, R. T. (2013) Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance, Antioxid. Redox Signal., 18, 1165-1207, https://doi.org/10.1089/ars.2011.4322.
Seco-Cervera, M., González-Cabo, P., Pallardó, F. V., Romá-Mateo, C., and García-Giménez, J. L. (2020) Thioredoxin and glutaredoxin systems as potential targets for the development of new treatments in Friedreich’s ataxia, Antioxidants (Basel), 9, 1257, https://doi.org/10.3390/antiox9121257.
Rhee, S. G., and Kil, I. S. (2017) Multiple functions and regulation of mammalian peroxiredoxins, Annu. Rev. Biochem., 86, 1-27, https://doi.org/10.1146/annurev-biochem-060815-014431.
Luo, W., Chen, I., Chen, Y., Alkam, D., Wang, Y., and Semenza, G. L. (2016) PRDX2 and PRDX4 are negative regulators of hypoxia-inducible factors under conditions of prolonged hypoxia, Oncotarget, 7, 6379-6397, https://doi.org/10.18632/oncotarget.7142.
Ma, S., Zhang, X., Zheng, L., Li, Z., Zhao, X., et al. (2016) Peroxiredoxin 6 is a crucial factor in the initial step of mitochondrial clearance and is upstream of the PINK1-Parkin pathway, Antioxid. Redox Signal., 24, 486-501, https://doi.org/10.1089/ars.2015.6336.
Sharapov, M. G., and Novoselov, V. I. (2019) Catalytic and signaling role of peroxiredoxins in carcinogenesis, Biochemistry (Moscow), 84, 79-100, https://doi.org/10.1134/S0006297919020019.
Portillo-Ledesma, S., Randall, L. M., Parsonage, D., Dalla Rizza, J., Karplus, P. A., et al. (2018) Differential kinetics of two-cysteine peroxiredoxin disulfide formation reveal a novel model for peroxide sensing, Biochemistry, 57, 3416-3424, https://doi.org/10.1021/acs.biochem.8b00188.
Kalinina, E. V., Chernov, N. N., and Novichkova, M. D. (2014) Role of glutathione, glutathione transferase, and glutaredoxin in regulation of redox-dependent processes, Biochemistry (Moscow), 79, 1562-1583, https://doi.org/10.1134/S0006297914130082.
Arakawa, S. (2013) Utilization of glutathione S-transferase Mu 1- and Theta 1-null mice as animal models for absorption, distribution, metabolism, excretion and toxicity studies, Expert Opin. Drug Metab. Toxicol., 9, 725-736, https://doi.org/10.1517/17425255.2013.780027.
Mohana, K., and Achary, A. (2017) Human cytosolic glutathione-S-transferases: quantitative analysis of expression, comparative analysis of structures and inhibition strategies of isozymes involved in drug resistance, Drug Metab. Rev., 49, 318-337, https://doi.org/10.1080/03602532.2017.1343343.
Singh, R. R., and Reindl, K. M. (2021) Glutathione S-transferases in cancer, Antioxidants (Basel), 10, 701, https://doi.org/10.3390/antiox10050701.
Yang, W. S., Sriramaratnam, R., Welsch, M. E., Shimada, K., Skouta, R., et al. (2014) Regulation of ferroptotic cancer cell death by GPX4, Cell, 156, 317-331, https://doi.org/10.1016/j.cell.2013.12.010.
Esworthy, R. S., Yang, L., Frankel, P. H., and Chu, F. F. (2005) Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice, J. Nutr., 135, 740-745, https://doi.org/10.1093/jn/135.4.740.
Chabory, E., Damon, C., Lenoir, A., Kauselmann, G., Kern, H., et al. (2009) Epididymis seleno-independent glutathione peroxidase 5 maintains sperm DNA integrity in mice, J. Clin. Invest., 119, 2074-2085, https://doi.org/10.1172/JCI38940.
Lu, L., Oveson, B. C., Jo, Y. J., Lauer, T. W., Usui, S., et al. (2009) Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage, Antioxid. Redox Signal., 11, 715-724, https://doi.org/10.1089/ars.2008.2171.
Brigelius-Flohé, R., and Flohé, L. (2020) Regulatory phenomena in the glutathione peroxidase superfamily, Antioxidants Redox Signal., 33, 498-516, https://doi.org/10.1089/ars.2019.7905.
Sharapov, M. G., Ravin, V. K., and Novoselov, V. I. (2014) Peroxiredoxins as multifunctional enzymes, Mol. Biol. (Mosk.), 48, 520-545, https://doi.org/10.1134/S0026893314040128.
Peskin, A. V., and Winterbourn, C. C. (2021) The enigma of 2-Cys peroxiredoxins: what are their roles? Biochemistry (Moscow), 86, 84-91, https://doi.org/10.1134/S0006297921010089.
Winterbourn, C. C., and Peskin, A. V. (2016) Kinetic approaches to measuring peroxiredoxin reactivity, Mol. Cells, 39, 26-30, https://doi.org/10.14348/molcells.2016.2325.
Flohé, L., Toppo, S., Cozza, G., and Ursini, F. (2011) A comparison of thiol peroxidase mechanisms, Antioxid. Redox Signal., 15, 763-780, https://doi.org/10.1089/ars.2010.3397.
Forshaw, T. E., Reisz, J. A., Nelson, K. J., Gumpena, R., Lawson, J. R., et al. (2021) Specificity of human sulfiredoxin for reductant and peroxiredoxin oligomeric state, Antioxidants (Basel), 10, 946, https://doi.org/10.3390/antiox10060946.
Liu, Y., Li, M., Du, X., Huang, Z., and Quan, N. (2021) Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases, Free Radic. Biol. Med., 163, 56-68, https://doi.org/10.1016/j.freeradbiomed.2020.11.015.
Fisher, A. B., Vasquez-Medina, J. P., Dodia, C., Sorokina, E. M., Tao, J.-Q., and Feinstein, S. I. (2018) Peroxiredoxin 6 phospholipid hydroperoxidase activity in the repair of peroxidized cell membranes, Redox Biol., 14, 41-46, https://doi.org/10.1016/j.redox.2017.08.008.
Perkins, A., Nelson, K. J., Parsonage, D., Poole, L. B., and Karplus, P. A. (2015) Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling, Trends Biochem Sci., 40, 435-445, https://doi.org/10.1016/j.tibs.2015.05.001.
Fisher, A. B. (2017) Peroxiredoxin 6 in the repair of peroxidized cell membranes and cell signaling, Arch. Biochem. Biophys., 617, 68-83, https://doi.org/10.1016/j.abb.2016.12.003.
Knoops, B., Becker, S., Poncin, M. A., Glibert, J., Derclaye, S., et al. (2018) Specific interactions measured by AFM on living cells between peroxiredoxin-5 and TLR4: relevance for mechanisms of innate immunity, Cell Chem. Biol., 25, 550-559.e3, https://doi.org/10.1016/j.chembiol.2018.02.006.
Sharapov, M. G., Glushkova, O. V., Parfenyuk, S. B., Gudkov, S. V., Lunin, S. M., and Novoselova, E. G. (2021) The role of TLR4/NF-κB signaling in the radioprotective effects of exogenous Prdx6, Arch. Biochem. Biophys., 702, 108830, https://doi.org/10.1016/j.abb.2021.108830.
Lee, Y. J. (2020) Knockout mouse models for peroxiredoxins, Antioxidants (Basel), 9, 182, https://doi.org/10.3390/antiox9020182.
Radyuk, S. N., and Orr, W. C. (2018) The multifaceted impact of peroxiredoxins on aging and disease, Antioxid. Redox Signal., 29, 1293-1311, https://doi.org/10.1089/ars.2017.7452.
Nelson, K. J., Perkins, A., Van Swearingen, A. E. D., Hartman, S., Brereton, A. E., et al. (2018) Experimentally dissecting the origins of peroxiredoxin catalysis, Antioxid. Redox Signal., 28, 521-536, https://doi.org/10.1089/ars.2016.6922.
Labunskyy, V. M., Hatfield, D. L., and Gladyshev, V. N. (2014) Selenoproteins: molecular pathways and physiological roles, Physiol. Rev., 94, 739-777, https://doi.org/10.1152/physrev.00039.2013.
Gladyshev, V. N., Kryukov, G. V., Fomenko, D. E., and Hatfield, D. L. (2004) Identification of trace element-containing proteins in genomic databases, Annu. Rev. Nutr., 24, 579-596, https://doi.org/10.1146/annurev.nutr.24.012003.132241.
Mariotti, M., Ridge, P. G., Zhang, Y., Lobanov, A. V., Pringle, T. H., et al. (2012) Composition and evolution of the vertebrate and mammalian selenoproteomes, PLoS One, 7, e33066, https://doi.org/10.1371/journal.pone.0033066.
Brigelius-Flohé, R., and Maiorino, M. (2013) Glutathione peroxidases, Biochim. Biophys. Acta Gen. Subj., 1830, 3289-3303, https://doi.org/10.1016/j.bbagen.2012.11.020.
Toppo, S., Flohé, L., Ursini, F., Vanin, S., and Maiorino, M. (2009) Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme, Biochim. Biophys. Acta Gen. Subj., 1790, 1486-1500, https://doi.org/10.1016/j.bbagen.2009.04.007.
Lubos, E., Loscalzo, J., and Handy, D. E. (2011) Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities, Antioxidants Redox Signal., 15, 1957-1997, https://doi.org/10.1089/ars.2010.3586.
Jiao, Y., Wang, Y., Guo, S., and Wang, G. (2017) Glutathione peroxidases as oncotargets, Oncotarget, 8, 80093-80102, https://doi.org/10.18632/oncotarget.20278.
Mannervik, B., Board, P. G., Hayes, J. D., Listowsky, I., and Pearson, W. R. (2005) Nomenclature for mammalian soluble glutathione transferases, Methods Enzymol., 401, 1-8, https://doi.org/10.1016/S0076-6879(05)01001-3.
Prohaska, J. R. (1980) The glutathione peroxidase activity of glutathione S-transferases, Biochim. Biophys. Acta, 611, 87-98, https://doi.org/10.1016/0005-2744(80)90045-5.
Lankin, V. Z., Tikhaze, A. K., Osis, Yu. G., Vikhert, A. M., Schewe, T., and Rapoport, S. (1985) Enzymatic regulation of lipid peroxidation in the membranes: the role of phospholipase A2 and glutathione transferase, Doklady Biochemistry, 281, 204-207.
Bondar’, T. N., Lankin, V. Z., and Antonovsky, V. L. (1989) The reduction of organic hydroperoxides by glutathione peroxidase and glutfthione S-transferase: the influence of substrate structure, Doklady Biochemistry, 304, 217-220.
Awasthi, Y. C., Zimniak, P., Singhal, S. S., and Awasthi, S. (1995) Physiological role of glutathione-S-transferases in protection mechanisms against lipid peroxidation: a commentary, Biochem. Arch., 11, 47-54.
Bao, Y., and Williamson, G. (1996) Metabolism of hydroperoxy-phospholipids in human hepatoma HepG2 cells, J. Lipid Res., 37, 2351-2360.
Lankin, V. Z., Bondar, T. N., and Tikhaze, A. K. (1997) The influence of free fatty acids on the lipoperoxidase activity of antioxidative enzymes-Se-containing glutathione peroxidase and nonselenic glutathione-S-transferase, Dokl. Akad. Nauk, 357, 828-831.
Sevanian, A., Muakkassah-Kelly, S. F., and Montestruque, S. (1983) The influence of phospholipase A2 and glutathione peroxidase on the elimination of membrane lipid peroxides, Arch. Biochem. Biophys., 223, 441-452, https://doi.org/10.1016/0003-9861(83)90608-2.
Lankin, V. Z., Tikhaze, A. K., and Osis, Y. G. (2002) Modeling the cascade of enzymatic reactions in liposomes including successive free radical peroxidation, reduction, and hydrolysis of phospholipid polyenoic acyls for studying the effect of these processes on the structural-dynamic parameters of the membranes, Biochemistry (Moscow), 67, 566-574, https://doi.org/10.1023/a:1015502429453.
Lankin, V. Z., Antonovsky, V. L., and Tikhaze, A. K. (2004) Regulation of free radical lipoperoxidation and organic peroxides metabolism during normal station and pathologies, in Peroxides at the Beginning of the Third Millennium, Nova Sci. Publ., pp. 85-111.
Lankin, V. Z., Tikhaze, A. K., Kapel’ko, V. I., Shepel’kova, G. S., Shumaev, K. B., et al. (2007) Mechanisms of oxidative modification of low density lipoproteins under conditions of oxidative and carbonyl stress, Biochemistry (Moscow), 72, 1081-1090, https://doi.org/10.1134/S0006297907100069.
Hayes, J. D., Flanagan, J. U., and Jowsey, I. R. (2005) Glutathione transferases, Annu. Rev. Pharmacol. Toxicol., 45, 51-88, https://doi.org/10.1146/annurev.pharmtox.45.120403.095857.
Allocati, N., Masulli, M., Di Ilio, C., and Federici, L. (2018) Glutathione transferases: substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases, Oncogenesis, 7, 8, https://doi.org/10.1038/s41389-017-0025-3.
Goncalves, M., Moura Neto, J., Souza, C., Melo, P., and Reis, M. (2010) Evaluating glutathione S-Transferase (GST) null genotypes (GSTT1 and GSTM1) as a potential biomarker of predisposition for developing leukopenia, Int. J. Lab. Hematol., 32, e49-e56, https://doi.org/10.1111/j.1751-553X.2009.01169.x.
Pljesa-Ercegovac, M., Savic-Radojevic, A., Matic, M., Coric, V., Djukic, T., et al. (2018) Glutathione transferases: potential targets to overcome chemoresistance in solid tumors, Int. J. Mol. Sci., 19, 3785, https://doi.org/10.3390/ijms19123785.
Acknowledgments
The authors express their gratitude to Dr. V. I. Novoselov for his invaluable comments on the manuscript.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 20-14-50114).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest in financial or any other sphere. This article does not contain description of studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Sharapov, M.G., Gudkov, S.V. & Lankin, V.Z. Hydroperoxide-Reducing Enzymes in the Regulation of Free-Radical Processes. Biochemistry Moscow 86, 1256–1274 (2021). https://doi.org/10.1134/S0006297921100084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921100084