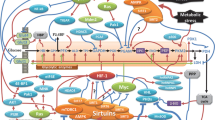
Abstract
According to modern concepts, tumor formation is associated with impairments in the structure of protooncogenes and/or deactivation of suppressor genes, regardless of the nature of carcinogenic factor. As a consequence, unregulated onco-proteins activate extracellular proteases, resulting in the destruction of the extracellular matrix, which facilitates cell invasion, deterioration of the cell-cell contacts, and metastasis. Tumor development requires activation of certain transcription factors; however, many oncoproteins are not transcription factors. It can be assumed that these oncoproteins are not the ultimate effectors of tumor development, but rather transmitters of the carcinogenic signal to the transcription factors promoting tumorigenesis. Here, we describe the mechanisms of carcinogenesis caused by various oncogenes/oncoproteins. We conclude that the common feature of these mechanisms is stimulation of aerobic glycolysis (Warburg effect) regulated, as a rule, through the activation of the HIFα transcription factor. The role of aerobic glycolysis at the early stages of carcinogenesis is discussed.
Similar content being viewed by others
Abbreviations
- ARNT:
-
aryl hydrocarbon receptor nuclear translocator
- MCT:
-
monocarboxylate transporter
- NOX:
-
NADPH oxidase complex
- ROS:
-
reactive oxygen species
References
Estrella, V., Chen, T., Lloyd, M., Wojtkowiak, J., Cornnell, H. H., Ibrahim-Hashim, A., Bailey, K., Balagurunathan, Y., Rothberg, J. M., Sloane, B. F., Johnson, J., Gatenby, R. A., and Gillies, R. J. (2013) Acidity generated by the tumor microenvironment drives local invasion, Cancer Res., 73, 1524–1535, doi: https://doi.org/10.1158/0008-5472.CAN-12-2796.
McCarty, M. F., and Whitaker, J. (2010) Manipulating tumor acidification as a cancer treatment strategy, Altern. Med. Rev., 15, 264–272.
Martin, N. K., Robey, I. F., Gaffney, E. A., Gillies, R. J., Gatenby, R. A., and Maini, P. K. (2012) Predicting the safety and efficacy of buffer therapy to raise tumor pHe: an integrative modelling study, Br. J. Cancer, 106, 1280–1287, doi: https://doi.org/10.1038/bjc.2012.58.
Harguindey, S., Arranz, J. L., Polo Orozco, J. D., Rauch, C., Fais, S., Cardone, R. A., and Reshkin, S. J. (2013). Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs - an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research, J. Transl. Med., 11, 282, doi: https://doi.org/10.1186/1479-5876-11-282.
Schlaepfer, I. R., Glode, L. M., Hitz, C. A., Pac, C. T., Boyle, K. E., Maroni, P., Deep, G., Agarwal, R., Lucia, S. M., Cramer, S. D., Serkova, N. J., and Eckel, R. H. (2015) Inhibition of lipid oxidation increases glucose metabolism and enhances 2-deoxy-2-[(18)F]fluoro-D-glucose uptake in prostate cancer mouse xenografts, Mol. Imaging Biol., 17, 529–538, doi: https://doi.org/10.1007/s11307-014-0814-4.
Guan, Z. W., Xu, B. X., Wang, R. M., Sun, L., and Tian, J. H. (2013) Hyperaccumulation of (18)F-FDG in order to differentiate solid pseudopapillary tumors from adenocar-cinomas and from neuroendocrine pancreatic tumors and review of the literature, Hell. J. Nucl. Med., 16, 97–102, doi: https://doi.org/10.1967/s002449910084.
Warburg, O., Posener, K., and Negelein, E. (1924) Uber den stoffwechsel der karzinomzellen, Biochemische Zeitschrift, 152, 309–344.
Pavlides, S., Whitaker-Menezes, D., Castello-Cros, R., Flomenberg, N., Witkiewicz, A. K., Frank, P. G., Casimiro, M. C., Wang, C., Fortina, P., Addya, S., Pestell, R. G., Martinez-Outschoorn, U. E., Sotgia, F., and Lisanti, M. P. (2009) The reverse Warburg effect: aerobic glycolysis in cancer-associated fibroblasts and the tumor stroma, Cell Cycle, 8, 3984–4001.
Yingqian, L. V., Shan, Z., Jinzhu, H., Likang, Z., Zixin, Y., and Zhao, L. (2015) Hypoxia-inducible factor-1α induces multidrug resistance protein in colon cancer, Onco Targets Ther., 8, 1941–1948, doi: https://doi.org/10.2147/OTT.S82835.
Krasnov, G. S., Dmitriev, A. A., and Lakunina, V. A. (2013) Targeting VDAC-bound hexokinase II: a promising approach for concomitant anti-cancer therapy, Expert Opin. Ther. Targets, 17, 1221–1230, doi: https://doi.org/10.1517/14728222.2013.833607.
Mazumdar, J., Dondeti, V., and Simon, M. C. (2009) Hypoxia-inducible factors in stem cells and cancer, J. Cell. Mol. Med., 13, 4319–4328, doi: https://doi.org/10.1111/j.1582-4934.2009.00963.x.
Axelson, H., Fredlund, E., Ovenberger, M., Landberg, G., and Pahlman, S. (2005) Hypoxia-induced dedifferentiation of tumor cells - a mechanism behind heterogeneity and aggressiveness of solid tumors, Semin. Cell Dev. Biol., 16, 554–563.
Marin-Hernandez, A., Gallardo-Perez, J. C., Ralph, S. J., Rodriguez-Enriquez, S., and Moreno-Sanchez, R. (2009) HIF-1α modulates energy metabolism in cancer cells by inducing overexpression of specific glycolytic isoforms, Mini Rev. Med. Chem., 9, 1084–1101.
Lou, F., Chen, X., Jalink, M., Zhu, Q., Ge, N., Zhao, S., Fang, X., Fan, Y., Bjorkholm, M., Liu, Z., and Xu, D. (2007) The opposing effect of hypoxia-inducible factor-2α on expression of telomerase reverse transcriptase, Mol. Cancer Res., 5, 793–800.
Khromova, N. V., Kopnin, P. B., Stepanova, E. V., Agapova, L. S., and Kopnin, B. P. (2009) p53 hot-spot mutants increase tumor vascularization via ROS-mediated activation of the HIF1/VEGF-A pathway, Cancer Lett., 276, 143–151, doi: https://doi.org/10.1016/j.canlet.2008.10.049.
Peng, X. H., Karna, P., Cao, Z., Jiang, B. H., Zhou, M., and Yang, L. (2006) Cross-talk between epidermal growth factor receptor and hypoxia-inducible factor-1alpha signal pathways increases resistance to apoptosis by up-regulating surviving gene expression, J. Biol. Chem., 281, 25903–25914.
Zhu, H., Wang, D., Liu, Y., Su, Z., Zhang, L., Chen, F., Zhou, Y., Wu, Y., Yu, M., Zhang, Z., and Shao, G. (2013) Role of the hypoxia-inducible factor-1alpha induced autophagy in the conversion of non-stem pancreatic cancer cells into CD133+ pancreatic cancer stem-like cells, Cancer Cell Int., 13, 119, doi: https://doi.org/10.1186/1475-2867-13-119.
Helczynska, K., Kronblad, A., Jogi, A., Nilsson, E., Beckman, S., and Landberg, G. (2003) Hypoxia promotes a dedifferentiated phenotype in ductal breast carcinoma in situ, Cancer Res., 63, 1441–1444.
Shin, D. H., Dier, U., Melendez, J. A., and Hempel, N. (2015) Regulation of MMP-1 expression in response to hypoxia is dependent on the intracellular redox status of metastatic bladder cancer cells, Biochim. Biophys. Acta, 1852, 2593–2602.
Erler, J. T., and Giaccia, A. J. (2006) Lysyl oxidase mediates hypoxic control of metastasis, Cancer Res., 66, 10238–10241.
Kobliakov, V. A. (2017) Role of proton pumps in tumorigenesis, Biochemistry (Moscow), 82, 401–412, doi: https://doi.org/10.1134/S0006297917040010.
Fukuda, R., Zhang, H., Kim, J. W., Shimoda, L., Dang, C. V., and Semenza, G. L. (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells, Cell, 129, 111–122.
Lee, K. A., Roth, R. A., and LaPres, J. J. (2007) Hypoxia, drug therapy and toxicity, Pharmacol. Ther., 113, 229–246, doi: https://doi.org/10.1016/j.pharmthera.2006.08.001.
Berra, E., Benizri, E., Ginouves, A., Volmat, V., Roux, D., and Pouyssegur, J. (2003) HIF prolylhydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia, Embo J., 22, 4082–4090.
Selak, M. A., Armour, S. M., MacKenzie, E. D., Boulahbel, H., Watson, D. G., Mansfield, K. D., Pan, Y., Simon, M. C., Thompson, C. B., and Gottlieb, E. (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase, Cancer Cell, 7, 77–85.
Chandel, N. S., McClintock, D. S., Feliciano, C. E., Wood, T. M., Melendez, J. A., Rodriguez, A. M., and Schumacker, P. T. (2000) Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing, J. Biol. Chem., 275, 25130–25138.
Lee, G., Won, H. S., Lee, Y. M., Choi, J. W., Oh, T. I., Jang, J. H., Choi, D. K., Lim, B. O., Kim, Y. J., Park, J. W., Puigserver, P., and Lim, J. H. (2016) Oxidative dimerization of PHD2 is responsible for its inactivation and contributes to metabolic reprogramming via HIF-1α activation, Sci. Rep., 6, 18928, doi: https://doi.org/10.1038/srep18928.
Cash, T. P., Pan, Y., and Simon, M. C. (2007) Reactive oxygen species and cellular oxygen sensing, Free Radic. Biol. Med., 43, 1219–1225.
Chandel, N. S., Maltepe, E., Goldwasser, E., Mathieu, C. E., Simon, M. C., and Schumacker, P. T. (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription, Proc. Natl. Acad. Sci. USA, 95, 11715–11720.
Mansfield, K. D., Guzy, R. D., Pan, Y., Young, R. M., Cash, T. P., Schumacker, P. T., and Simon, M. C. (2005) Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation, Cell Metab., 1, 393–399.
Bell, E. L., Klimova, T. A., Eisenbart, J., Moraes, C. T., Murphy, M. P., Budinger, G. R., and Chandel, N. S. (2007) The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production, J. Cell Biol., 177, 1029–1036.
Henegan, J. C., Jr., and Gomez, C. R. (2016) Heritable cancer syndromes related to the hypoxia pathway, Front. Oncol., 6, 68, doi: https://doi.org/10.3389/fonc.2016.00068.
Yang, H., and Kaelin, W. G., Jr. (2001) Molecular pathogenesis of the von Hippel-Lindau hereditary cancer syndrome: implications for oxygen sensing, Cell Growth Differ., 12, 447–455.
hao, T., Mu, X., and You, Q. (2017) Succinate: an initiator in tumorigenesis and progression, Oncotarget, 8, 53819–53828, doi: https://doi.org/10.18632/oncotarget.17734.
Luo, W., Hu, H., Chang, R., Zhong, J., Knabel, M., O’Meally, R., Cole, R. N., Pandey, A., and Semenza, G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1, Cell, 145, 732–744.
De Saedeleer, C. J., Copetti, T., Porporato, P. E., Verrax, J., Feron, O., and Sonveaux, P. (2012) Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells, PLoS One, 7, e46571, doi: https://doi.org/10.1371/journal.pone.0046571.
Warfel, N. A., Dolloff, N. G., Dicker, D. T., Malysz, J., and El-Deiry, W. S. (2013) CDK1 stabilizes HIF-1α via direct phosphorylation of Ser668 to promote tumor growth, Cell Cycle, 12, 3689–3701.
Hubbi, M. E., Gilkesa, D. M., Hu, H., Ahmede, I., and Semenza, G. L. (2014) Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1α to promote cell-cycle progression, Proc. Natl. Acad. Sci. USA, 111, E3325–E3334.
Kietzmann, T. (2017) Metabolic zonation of the liver: the oxygen gradient revisited, Redox Biol., 11, 622–630.
Phan, A. T., and Goldrath, A. W. (2015) Hypoxia-inducible factors regulate T cell metabolism and function, Mol. Immunol., 68, 527–535, doi: https://doi.org/10.1016/j.molimm.2015.08.004.
Michalekand, R. D., and Rathmell, J. C. (2010) The metabolic life and times of a T-cell, Immunol. Rev., 236, 190–202, doi: https://doi.org/10.1111/j.1600-065X.2010.00911.x.
Ohshima, H., Tatemichi, M., and Sawa, T. (2003) Chemical basis of inflammation-induced carcinogenesis, Arch. Biochem. Biophys., 417, 3–11.
Schwartsburd, P. M. (2003) Chronic inflammation as inductor of pro-cancer microenvironment: pathogenesis of dysregulated feedback control, Cancer Metastasis Rev., 22, 95–102.
Bokoch, G. M., and Knaus, U. G. (2003) NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci., 28, 502–508.
Morry, J., Ngamcherdtraku, W., and Yantasee, W. (2017) Oxidative stress in cancer and fibrosis: opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles, Redox Biol., 11, 240–253, doi: https://doi.org/10.1016/j.redox.2016.12.011.
Balamurugan, K. (2016) HIF-1 at the crossroads of hypoxia, inflammation, and cancer, Int. J. Cancer, 138, 1058–1066.
Block, K., Gorin, Y., Hoover, P., Williams, P., Chelmicki, T., Clark, R. A., Yoneda, T., and Abboud, H. E. (2007) NAD(P)H oxidases regulate HIF-2alpha protein expression, J. Biol. Chem., 282, 8019–8026.
Juhasz, A., Markel, S., Gaur, S., Liu, H., Lu, J., Jiang, G., Wu, X., Antony, S., Wu, Y., Melillo, G., Meitzler, J. L., Haines, D. C., Butcher, D., Roy, K., and Doroshow, J. H. (2017) NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction, J. Biol. Chem., 292, 7866–7887.
Antony, S., Jiang, G., Wu, Y., Meitzler, J. L., Makhlouf, H. R., Haines, D. C., Butcher, D., Hoon, D. S., Ji, J., Zhang, Y., Juhasz, A., Lu, J., Liu, H., Dahan, I., Konate, M., Roy, K. K., and Doroshow, J. H. (2017) NADPH oxidase 5 (NOX5)-induced reactive oxygen signaling modulates nor-moxic HIF-1α and p27Kip1 expression in malignant melanoma and other human tumors, Mol. Carcinog., 56, 2643–2662.
Skonieczna, M., Hejmo, T., Poterala-Hejmo, A., Cieslar-Pobuda, A., and Buldak, R. J. (2017) NADPH oxidases: insights into selected functions and mechanisms of action in cancer and stem cells, Oxid. Med. Cell. Longev., 2017, 9420539, doi: https://doi.org/10.1155/2017/9420539.
Baeuerle, P. A., and Baltimore, D. (1996) NF-kappa B: ten years after, Cell, 87, 13–20.
D’Ignazio, L., Bandarra, D., and Rocha, S. (2016) NF-κB and HIF crosstalk in immune responses, FEBS J., 283, 413–424, doi: https://doi.org/10.1111/febs.13578.
Remels, A. H., Gosker, H. R., Verhees, K. J., Langen, R. C., and Schols, A. M. (2015) TNF-α-induced NF-κB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1α, Endocrinology, 156, 1770–1781.
Pylayeva-Gupta, Y., Grabocka, E., and Bar-Sagi, D. (2011) RAS oncogenes: weaving a tumorigenic web, Nat. Rev. Cancer, 11, 761–774.
Scheffzek, K., Ahmadian, M. R., Kabsch, W., Wiesmuller, L., Lautwein, A., Schmitz, F., and Wittinghofer, A. (1997) The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants, Science, 277, 333–338.
Bryant, K. L., Mancias, J. D., Kimmelman, A. C., and Der, C. J. (2014) KRAS: feeding pancreatic cancer proliferation, Trends Biochem. Sci., 39, 91–100, doi: https://doi.org/10.1016/j.tibs.2013.12.004.
Hu, Y., Lu, W., Chen, G., Wang, P., Chen, Z., Zhou, Y., Ogasawara, M., Trachootham, D., Feng, L., and Pelicano, H. (2012) K-ras(G12V) transformation leads to mitochon-drial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis, Cell Res., 22, 399–412.
Chesney, J., and Telang, S. (2013) Regulation of glycolytic and mitochondrial metabolism by ras, Curr. Pharm. Biotechnol., 14, 251–260.
Irani, K., Xia, Y., Zweier, J. L., Sollott, S. J., Der, C. J., Fearon, E. R., Sundaresan, M., Finkel, T., and Goldschmidt-Clermont, P. J. (1997) Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts, Science, 275, 1649–1652.
Mitsushita, J., Lambeth, J. D., and Kamata, T. (2004) The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation, Cancer Res., 64, 3580–3585.
Komatsu, D., Kato, M., Nakayama, J., Miyagawa, S., and Kamata, T. (2008) NADPH oxidase 1 plays a critical mediating role in oncogenic Ras-induced vascular endothelial growth factor expression, Oncogene, 27, 4724–4732, doi: https://doi.org/10.1038/onc2008.102.
Qiu, R. G., Chen, J., Kirn, D., and Moon, A. (1995) An essential role for Rac in Ras transformation, Nature, 374, 457–459.
Kissil, J. L., Walmsley, M. J., Hanlon, L., Haigis, K. M., Bender Kim, C. F., Sweet-Cordero, A., Eckman, M. S., Tuveson, D. A., Capobianco, A. J., Tybulewicz, V. L., and Jacks, T. (2007) Requirement for Rac1 in a K-ras induced lung cancer in the mouse, Cancer Res., 67, 8089–8094.
Kazanietz, M. G., and Caloca, M. J. (2017) The Rac GTPase in cancer: from old concepts to new paradigms, Cancer Res., 77, 5445–5451.
Malliri, A., van Der Kammen, R. A., Clark, K., van der Valk, M., Michiels, F., and Collard, J. G. (2002) Mice deficient in the Rac activator Tiam1 are resistant to Rasinduced skin tumors, Nature, 417, 867–871.
Adachi, Y., Shibai, Y., Mitsushita, J., Shang, W. H., Hirose, K., and Kamata, T. (2008) Oncogenic Ras upregulates NADPH oxidase 1 gene expression through MEK-ERK-dependent phosphorylation of GATA-6, Oncogene, 27, 4921–4932.
Wu, R. F., and Terada, L. S. (2009) Ras and Nox: linked signaling networks? Free Radic. Biol. Med., 47, 1276–1281, doi: https://doi.org/10.1016/j.freeradbiomed.2009.05.037.
Neuzil, J., Rohlena, J., and Dong, L. F. (2012) K-Ras and mitochondria: dangerous liaisons, Cell Res., 22, 285–287, doi: https://doi.org/10.1038/cr.2011.160.
Weinberg, F., Hamanaka, R., Wheaton, W. W., Weinberg, S., Joseph, J., Lopez, M., Kalyanaraman, B., Mutlu, G. M., Budinger, G. R., and Chandel, N. S. (2010) Mitochondrial metabolism and ROS generation are essential for K-ras-mediated tumorigenicity, Proc. Natl. Acad. Sci. USA, 107, 8788–8793.
Martin, T. D., Cook, D. R., Choi, M. Y., Li, M. Z., Haigis, K. M., and Elledge, S. J. (2017) Role for mitochondrial translation in promotion of viability in K-Ras mutant cells, Cell Rep., 20, 427–438.
Heit, B., Yeung, T., and Grinstein, S. (2011) Changes in mitochondrial surface charge mediate recruitment of signaling molecules during apoptosis, Am. J. Physiol. Cell. Physiol., 300, C33–C41.
Irby, R. B., and Yeatman, T. J. (2000) Role of Src expression and activation in human cancer, Oncogene, 19, 5636–5642.
Siveen, K. S., Prabhu, K. S., Achkar, I. W., Kuttikrishnan, S., Shyam, S., Khan, A. Q., Merhi, M., Dermime, S., and Uddin, S. (2018) Role of non-receptor tyrosine kinases in hematological malignances and its targeting by natural products, Mol. Cancer, 17, 31, doi: https://doi.org/10.1186/s12943-018-0788-y.
Carroll, R. C., Ash, J. F., Vogt, P. K., and Singer, S. J. (1978) Reversion of transformed glycolysis to normal by inhibition of protein synthesis in rat kidney cells infected with temperature-sensitive mutant of Rous sarcoma virus, Proc. Natl. Acad. Sci. USA, 75, 5015–5019.
Lee, H. Y., Lee, T., and Lee, N. (2011) Src activates HIF1α not through direct phosphorylation of HIF1α specific prolyl-4 hydroxylase 2 but through activation of the NADPH oxidase/Rac pathway, Carcinogenesis, 32, 703–712.
Jin, Y., Cai, Q., Shenoy, A. K., Lim, S., Zhang, Y., Charles, S., Tarrash, M., Fu, X., Kamarajugadda, S., Trevino, J. G., Tan, M., and Lu, J. (2016) Src drives the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase through tyrosine-289 phosphorylation, Oncotarget, 7, 25113–25124.
Chou, M. T., Anthony, J., Bjorge, J. D., and Fujita, D. J. (2010) The von Hippel-Lindau tumor suppressor protein is destabilized by Src: implications for tumor angiogenesis and progression, Genes Cancer, 1, 225–238.
Vettori, A., Greenald, D., Wilson, G. K., Peron, M., Facchinello, N., Markham, E., Sinnakaruppan, M., Matthews, L. C., McKeating, J. A., Argenton, F., and van Eeden, F. J. M. (2017) Glucocorticoids promote Von Hippel-Lindau degradation and Hif1α stabilization, Proc. Natl. Acad. Sci. USA, 114, 9948–9953.
Leontieva, O. V., Demidenko, Z. N., and Blagosklonny, M. V. (2015) Dual mTORC1/C2 inhibitors suppress cellular geroconversion (a senescence program), Oncotarget, 6, 23238–23248.
Leontieva, O. V., and Blagosklonny, M. V. (2014) M-TOR of pseudo-hypoxic state in aging: rapamycin to the rescue, Cell Cycle, 13, 509–515.
Sato, T., Nakashima, A., Guo, L., Coffman, K., and Tamanoi, F. (2010) Single amino-acid changes that confer constitutive activation of mTOR are discovered in human cancer, Oncogene, 29, 2746–2752.
Grabiner, B. C., Nardi, V., Birsoy, K., Possemato, R., Shen, K., Sinha, S., Jordan, A., Beck, A. H., and Sabatini, D. M. (2014) A diverse array of cancer-associated mTOR mutations are hyperactivating and can predict rapamycin sensitivity, Cancer Discov., 4, 554–563.
Blagosklonny, M. V. (2008) Prevention of cancer by inhibiting aging, Cancer Biol. Ther., 7, 1520–1524.
Ma, X. M., and Blenis, J. (2009) Molecular mechanisms of mTOR-mediated translational control, Nat. Rev. Mol. Cell Biol., 10, 307–318, doi: https://doi.org/10.1038/nrm2672.
Busch, S., Renaud, S. J., Schleussner, E., Graham, C. H., and Markert, U. R. (2009) mTOR mediates human trophoblast invasion through regulation of matrix-remodeling enzymes and is associated with serine phosphorylation of STAT3, Exp. Cell Res., 315, 1724–1733, doi: https://doi.org/10.1016/j.yexcr.2009.01.026.
Dodd, K., Yang, J., Shen, M., Sampson, J., and Tee, A. (2015) mTORC1 drives HIF-1α and VEGF-A signaling via multiple mechanisms involving 4E-BP1, S6K1 and STAT3, Oncogene, 34, 2239–2250.
Gao, P., Niu, N., Wei, T., Tozawa, H., Chen, X., Zhang, C., Zhang, J., Wada, Y., Kapron, C. M., and Liu, J. (2017) The roles of signal transducer and activator of transcription factor 3 in tumor angiogenesis, Oncotarget, 8, 69139–69161.
Shaw, R. J. (2009) LKB1 and AMP-activated protein kinase control of mTOR signaling and growth, Acta Physiol. (Oxf.), 196, 65–80.
Kim, I. Y., and Yu-Ying, H. (2013) Targeting the AMP-activated protein kinase for cancer prevention and therapy, Front. Oncol., 3, 175, doi: https://doi.org/10.3389/fonc.2013.00175.
Faubert, B., Boily, G., Izreig, S., Griss, T., Samborska, B., Dong, Z., Dupuy, F., Chambers, C., Fuerth, B. J., Viollet, B., Mamer, O. A., Avizonis, D., DeBerardinis, R. J., Siegel, P. M., and Jones, R. G. (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo, Cell Metab., 17, 113–124.
Schmelzle, T., and Hall, M. (2000) TOR, a central controller, Cell, 103, 253–262.
Kim, E. K., Yun, S. J., Ha, J. M., Kim, Y. W., Jin, I. H., Yun, J., Shin, H. K., Song, S. H., Kim, J. H., Lee, J. S., Kim, C. D., and Bae, S. S. (2011) Selective activation of Akt1 by mammalian target of rapamycin complex 2 regulates cancer cell migration, invasion, and metastasis, Oncogene, 30, 2954–2963.
Prendergast, G. C. (1999) Mechanisms of apoptosis by c-Myc, Oncogene, 18, 2967–2987.
Yoshida, G. J. (2018) Emerging roles of Myc in stem cell biology and novel tumor therapies, J. Exp. Clin. Cancer Res., 37, 173, doi: https://doi.org/10.1186/s13046-018-0835-y.
Wahlstrom, T., and Henriksson, M. A. (2015) Impact of MYC in regulation of tumor cell metabolism, Biochim. Biophys. Acta, 1849, 563–569.
Kalkat, M., De Melo, J., Hickman, K. A., Lourenco, C., Redel, C., Resetca, D., Tamachi, A., Tu, W. B., and Penn, L. Z. (2017) MYC deregulation in primary human cancers, Genes (Basel), 8, E151, doi: https://doi.org/10.3390/genes8060151.
Schaub, F. X., Dhankani, V., Berger, A. C., Trivedi, M., Richardson, A. B., Shaw, R., Zhao, W., Zhang, X., Ventura, A., Liu, Y., Ayer, D. E., Hurlin, P. J., Cherniack, A. D., Eisenman, R. N., Bernard, B., and Grandori, C. (2018) Pan-cancer alterations of the MYC oncogene and its proximal network across the cancer genome atlas, Cell Syst., 6, 282–300.
Dang, C. V. (2012) MYC on the path to cancer, Cell, 149, 22–35.
Osthus, R. C., Shim, H., Kim, S., Li, Q., Reddy, R., Mukherjee, M., Xu, Y., Wonsey, D., Lee, L. A., and Dang, C. V. (2000) Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc, J. Biol. Chem., 275, 21797–21800.
He, T. L., Zhang, Y. J., Jiang, H., Li, X. H., Zhu, H., and Zheng, K. L. (2015) The c-Myc-LDHA axis positively regulates aerobic glycolysis and promotes tumor progression in pancreatic cancer, Med. Oncol., 32, 187, doi: https://doi.org/10.1007/s12032-015-0633-8.
Kim, J. W., Gao, P., Liu, Y. C., Semenza, G. L., and Dang, C. V. (2007) Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1, Mol. Cell. Biol., 27, 7381–7393.
Zarrabi, A. J., Kao, D., Nguyen, D. T., Loscalzo, J., and Handy D. E. (2017) Hypoxia-induced suppression of c-Myc by HIF-2α in human pulmonary endothelial cells attenuates TFAM expression, Cell. Signal., 38, 230–237.
Huang, L. E. (2008) Carrot and stick: HIF-alpha engages c-Myc in hypoxic adaptation, Cell Death Differ., 15, 672–677, doi: https://doi.org/10.1038/sj.cdd.4402302.
Goda, N., Ryan, H. E., Khadivi, B., McNulty, W., Rickert, R. C., and Johnson, R. S. (2003) Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia, Mol. Cell. Biol., 23, 359–369.
Koshiji, M., Kageyama, Y., Pete, E. A., Horikawa, I., Barrett, J. C., and Huang, L. E. (2004) HIF-1alpha induces cell cycle arrest by functionally counteracting Myc, EMBO J., 23, 1949–1956.
Wang, L., Xue, M., and Chung, D. C. (2016) c-Myc is regulated by HIF-2α in chronic hypoxia and influences sensitivity to 5-FU in colon cancer, Oncotarget, 7, 78910–78917.
Dang, C. V., Kim, J. W., Gao, P., and Yustein, J. (2008) The interplay between MYC and HIF in cancer, Nat. Rev. Cancer, 8, 51–56.
Zhou, Q., Jin, P., Liu, J., Wang, F., and Xi, S. (2018) HER2 and Src co-regulate proliferation, migration and transformation by downstream signaling pathways in arsenite-treated human uroepithelial cells, Metallomics, 10, 1141–1159.
Wang, L. H., Jiang, X. R., Yang, J. Y., Bao, X. F., Chen, J. L., Liu, X., Chen, G. L., and Wu, C. F. (2016) SYP-5, a novel HIF1 inhibitor, suppresses tumor cells invasion and angiogenesis, Eur. J. Pharmacol., 791, 560–568.
Niu, W., Luo, Y., Wang, X., Zhou, Y., Li, H., Wang, H., Fu, Y., Liu, S., Yin, S., Li, J., Zhao, R., Liu, Y., Fan, S., Li, Z., Xiong, W., Li, X., Li, G., Ren, C., Tan, M., and Zhou, M. (2018) BRD7 inhibits the Warburg effect and tumor progression through inactivation of HIF1α/LDHA axis in breast cancer, Cell Death Dis., 9, 519, doi: https://doi.org/10.1038/s41419-018-0536-7.
Semenza, G. L. (2009) Regulation of cancer cell metabolism by hypoxia-inducible factor 1, Seminars in Cancer Biology, 19, 12–16.
Masoud, G. N., and Li, W. (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy, Acta Pharm. Sin. B, 5, 378-389, doi: https://doi.org/10.1016/j.apsb.2015.05.007.
Park, K., Lee, H. E., Lee, S. H., Lee, D., Lee, T., and Lee, Y. M. (2017) Molecular and functional evaluation of a novel HIF inhibitor, benzopyranyl 1,2,3-triazole compound, Oncotarget, 8, 7801–7813.
Masoud, G. N., Wang, J., Chen, J., Miller, D., and Li, W. (2015) Design, synthesis and biological evaluation of novel HIF1α inhibitors, Anticancer Res., 35, 3849–3859.
Funding
The work was carried out and financed within the budgetary project “Genotoxic action of chemotherapeutical preparations on medical staff during the treatment of oncology patients” (AAAA-A19-119031390107-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The author declares no conflict of interest.
Compliance with ethical norms. This article does not contain description of studies with participation of humans or animals performed by the author.
Additional information
Russian Text © The Author(s), 2019, published in Biokhimiya, 2019, Vol. 84, No. 10, pp. 1371-1384.
Rights and permissions
About this article
Cite this article
Kobliakov, V.A. The Mechanisms of Regulation of Aerobic Glycolysis (Warburg Effect) by Oncoproteins in Carcinogenesis. Biochemistry Moscow 84, 1117–1128 (2019). https://doi.org/10.1134/S0006297919100018
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297919100018