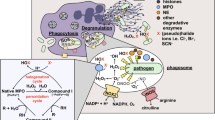
Abstract
Neutrophil myeloperoxidase (MPO) plays an important role in protecting the body against infections. MPO products–hypohalous acids and phenoxyl radicals–are strong oxidants that can damage not only foreign intruders but also host tissues, including blood plasma proteins. Here, we compared the MPO-induced oxidation of two plasma proteins with antioxidant properties–human serum albumin (HSA) and ceruloplasmin (CP). Incubation of both proteins with hypochlorite (NaOCl) or catalytically active MPO (MPO + H2O2), which synthesizes hypochlorous acid (HOCl) in the presence of chloride ions, resulted in the quenching of protein tryptophan fluorescence. Oxidation-induced changes in the structures of HSA and CP were different. HSA efficiently neutralized MPO-generated oxidants without protein aggregation, while CP oxidation resulted in the formation of large aggregates stabilized by strong covalent bonds between the aromatic amino acid residues. Tyrosine is present in the plasma as free amino acid and also as a component of the polypeptide chains of the proteins. The number of tyrosine residues in a protein does not determine its propensity for aggregate formation. In the case of C P, protein aggregation was primarily due to the high content of tryptophan residues in its polypeptide chain. MPO-dependent oxidation of free tyrosine results in the formation of tyrosyl radicals, that do not oxidize aromatic amino acid residues in proteins because of the high rate of recombination with dityrosine formation. At the same time, free tyrosine can influence MPO-induced protein oxidation due to its ability to modulate HOCl synthesis in the MPO active site.
Similar content being viewed by others
Abbreviations
- CP:
-
ceruloplasmin
- MPO:
-
myeloperoxidase
- Tyr:
-
tyrosine
References
Arnhold, J. (2004) Free radicals–friends or foes? Properties, functions, and secretion of human myeloperoxidase, Biochemistry (Moscow), 69, 4–9, doi: 10.1023/B:BIRY.0000016344.59411.ee.
Davies, M. J., Hawkins, C. L., Pattison, D. I., and Rees, M. D. (2008) Mammalian heme peroxidases: from molecular mechanisms to health implications, Antioxid. Redox Signal., 10, 1199–1234, doi: https://doi.org/10.1089/ars.2007.1927.
Arnhold, J., Furtmuller, P. G., and Obinger, C. (2003) Redox properties of myeloperoxidase, Redox Rep., 8, 179–186, doi: https://doi.org/10.1179/135100003225002664.
Furtmuller, P. G., Burner, U., Jantschko, W., Regelsberger, G., and Obinger, C. (2000) Two-electron reduction and one-electron oxidation of organic hydroperoxides by human myeloperoxidase, FEBS Lett., 484, 139–143.
Kirchner, T., Flemmig, J., Furtmьller, P. G., Obinger, C., and Arnhold, J. (2010) (–)-Epicatechin enhances the chlorinating activity of human myeloperoxidase, Arch. Biochem. Biophys., 495, 21–27, doi: https://doi.org/10.1016/j.abb.2009.12.013.
Flemmig, J., Remmler, J., Rohring, F., and Arnhold, J. (2014) (–)-Epicatechin regenerates the chlorinating activity of myeloperoxidase in vitro and in neutrophil granulocytes, J. Inorg. Biochem., 130, 84–91, doi: https://doi.org/10.1016/j.jinorgbio.2013.10.002.
Vlasova, I. I., Sokolov, A. V., and Arnhold, J. (2012) The free amino acid tyrosine enhances the chlorinating activity of human myeloperoxidase, J. Inorg. Biochem., 106, 76–83, doi: https://doi.org/10.1016/j.jinorgbio.2011.09.018.
Tzikas, S., Schlak, D., Sopova, K., Gatsiou, A., Stakos, D., Stamatelopoulos, K., Stellos, K., and Laske, C. (2014) Increased myeloperoxidase plasma levels in patients with Alzheimer’s disease, J. Alzheimer’s Dis., 39, 557–564, doi: https://doi.org/10.3233/JAD-131469.
Baldus, S., Heeschen, C., Meinertz, T., Zeiher, A. M., Eiserich, J. P., Munzel, T., Simoons, M. L., and Hamm, C. W. (2003) Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes, Circulation, 108, 1440–1445, doi: 10.1161/01.CIR.0000090690.67322.51.
Vlasova, I. I., Arnhold, J., Osipov, A. N., and Panasenko, O. M. (2006) pH-dependent regulation of myeloperoxidase activity, Biochemistry (Moscow), 71, 667–677.
Furtmuller, P. G., Zederbauer, M., Jantschko, W., Helm, J., Bogner, M., Jakopitsch, C., and Obinger, C. (2006) Active site structure and catalytic mechanisms of human peroxidases, Arch. Biochem. Biophys., 445, 199–213, doi: https://doi.org/10.1016/j.abb.2005.09.017.
Ramos, D. R., Garcia, M. V., Canle, L. M., Santaballa, J. A., Furtmuller, P. G., and Obinger, C. (2008) Myeloperoxidase-catalyzed chlorination: the quest for the active species, J. Inorg. Biochem., 102, 1300–1311, doi: https://doi.org/10.1016/j.jinorgbio.2008.01.003.
Zhang, R., Brennan, M. L., Shen, Z., MacPherson, J. C., Schmitt, D., Molenda, C. E., and Hazen, S. L. (2002) Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation, J. Biol. Chem., 277, 46116–46122, doi: https://doi.org/10.1074/jbc.M209124200.
Vlasova, I. I., Feng, W.-H., Goff, J. P., Giorgianni, A., Do, D., Gollin, S. M., Lewis, D. W., Kagan, V. E., and Yalowich, J. C. (2011) Myeloperoxidase-dependent oxidation of etoposide in human myeloid progenitor CD34+ cells, Mol. Pharmacol., 79, 479–487, doi: https://doi.org/10.1124/mol.110.068718.
Jantschko, W., Furtmuller, P. G., Zederbauer, M., Neugschwandtner, K., Lehner, I., Jakopitsch, C., Arnhold, J., and Obinger, C. (2005) Exploitation of the unusual thermodynamic properties of human myeloperoxidase in inhibitor design, Biochem. Pharmacol., 69, 1149–1157, doi: https://doi.org/10.1016/j.bcp.2005.02.006.
Pattison, D. I., and Davies, M. J. (2006) Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases, Curr. Med. Chem., 13, 3271–3290, doi: https://doi.org/10.2174/092986706778773095.
Senthilmohan, R., and Kettle, A. J. (2006) Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride, Arch. Biochem. Biophys., 445, 235–244, doi: https://doi.org/10.1016/j.abb.2005.07.005.
Brennan, M. L., and Hazen, S. L. (2003) Amino acid and protein oxidation in cardiovascular disease, Amino Acids, 25, 365–374, doi: https://doi.org/10.1007/s00726-003-0023-y.
Shao, B., Tang, C., Sinha, A., Mayer, P. S., Davenport, G. D., Brot, N., Oda, M. N., Zhao, X. Q., and Heinecke, J. W. (2014) Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase, Circ. Res., 114, 1733–1742, doi: 10.1161/CIRCRESAHA.114.303454.
Arnhold, J., Hammerschmidt, S., Wagner, M., Mueller, S., Arnold, K., and Grimm, E. (1990) On the action of hypochlorite on human serum albumin, Biomed. Biochim. Acta, 49, 991–997.
Colombo, G., Clerici, M., Altomare, A., Rusconi, F., Giustarini, D., Portinaro, N., Garavaglia, M. L., Rossi, R., Dalle-Donne, I., and Milzani, A. (2017) Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid, J. Proteomics, 152, 22–32, doi: https://doi.org/10.1016/j.jprot.2016.10.008.
Colombo, G., Reggiani, F., Cucchiari, D., Portinaro, N. M., Giustarini, D., Rossi, R., Garavaglia, M. L., Saino, N., Milzani, A., Badalamenti, S., and Dalle-Donne, I. (2017) Plasma protein-bound di-tyrosines as biomarkers of oxidative stress in end stage renal disease patients on maintenance haemodialysis, BBA Clin., 7, 55–63, doi: https://doi.org/10.1016/j.bbacli.2016.12.004.
Meotti, F. C., Jameson, G. N. L., Turner, R., Harwood, D. T., Stockwell, S., Rees, M. D., Thomas, S. R., and Kettle, A. J. (2011) Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation, J. Biol. Chem., 286, 12901–12911, doi: https://doi.org/10.1074/jbc.M110.172460.
Salavej, P., Spalteholz, H., and Arnhold, J. (2006) Modification of amino acid residues in human serum albumin by myeloperoxidase, Free Radic. Biol. Med., 40, 516–525, doi: https://doi.org/10.1016/j.freeradbiomed.2005.09.007.
Carr, A. C., McCall, M. R., and Frei, B. (2000) Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection, Arterioscler. Thromb. Vasc. Biol., 20, 1716–1723, doi: 0.1161/01.ATV.20.7.1716.
Dobretsov, G. E., Syrejshchikova, T. I., Smolina, N. V., and Uzbekov, M. V. (2015) CAPIDAN, a fluorescent reporter for detection of albumin drug-binding site changes, in Human Serum Albumin (HSA) (Stokes T., ed.) Nova Science Publisher, Inc., pp. 129–171.
Colombo, G., Clerici, M., Giustarini, D., Rossi, R., Milzani, A., and Dalle-Donne, I. (2012) Redox albuminomics: oxidized albumin in human diseases, Antioxid. Redox Signal., 17, 1515–1527, doi: https://doi.org/10.1089/ars.2012.4702.
Sozarukova, M. M., Proskurnina, E. V., and Vladimirov, Yu. A. (2016) Serum albumin as a sourse of and a target for free radicals in pathology, Bull. RSMU, 1, 56–61.
Torres, M. J., Turell, L., Botti, H., Antmann, L., and Carballal, S. (2012) Modulation of the reactivity of the thiol of human serum albumin and its sulfenic derivative by fatty acids, Arch. Biochem. Biophys., 521, 102–110, doi: https://doi.org/10.1016/j.abb.2012.03.011.
Tiruppathi, C., Naqvi, T., Wu, Y., Vogel, S. M., Minshall, R. D., and Malik, A. B. (2004) Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells, Proc. Natl. Acad. Sci. USA, 101, 7699–7704, doi: https://doi.org/10.1073/pnas.0401712101.
Atanasiu, R. L., Stea, D., Mateescu, M. A., Vergely, C., Dalloz, F., Briot, F., Maupoil, V., Nadeau, R., and Rochette, L. (1998) Direct evidence of caeruloplasmin antioxidant properties, Mol. Cell. Biochem., 189, 127–135.
Barinov, N. A., Vlasova, I. I., Sokolov, A. V., Kostevich, V. A., Dubrovin, E. V., and Klinov, D. V. (2018) High-resolution atomic force microscopy visualization of metalloproteins and their complexes, Biochim. Biophys. Acta Gen. Subj., 1862, 2862–2868, doi: https://doi.org/10.1016/j.bbagen.2018.09.008.
Sokolov, A., Ageeva, K., Pulina, M., Cherkalina, O., Samygina, V., Vlasova, I. I., Panasenko, O., Zakharova, E., and Vasilyev, V. (2008) Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other, Free Radic. Res., 42, 989–998, doi: https://doi.org/10.1080/10715760802566574.
Griffin, S. V., Chapman, P. T., Lianos, E. A., and Lockwood, C. M. (1999) The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies, Kidney Int., 55, 917–925, doi: https://doi.org/10.1046/j.1523-1755.1999.055003917.x.
Park, Y. S., Suzuki, K., Mumby, S., Taniguchi, N., and Gutteridge, J. M. (2000) Antioxidant binding of caeruloplasmin to myeloperoxidase: myeloperoxidase is inhibited, but oxidase, peroxidase and immunoreactive properties of caeruloplasmin remain intact, Free Radic. Res., 33, 261–265.
Chapman, A. L. P., Mocatta, T. J., Shiva, S., Seidel, A., Chen, B., Khalilova, I., Paumann-Page, M. E., Jameson, G. N. L., Winterbourn, C. C., and Kettle, A. J. (2013) Ceruloplasmin is an endogenous inhibitor of myeloperoxidase, J. Biol. Chem., 288, 6465–6477, doi: https://doi.org/10.1074/jbc.M112.418970.
Segelmark, M., Persson, B., Hellmark, T., and Wieslander, J. (1997) Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin. Exp. Immunol., 108, 167–174.
Sokolov, A. V., Pulina, M. O., Ageeva, K. V., Ayrapetov, M. I., Berlov, M. N., Volgin, G. N., Markov, A. G., Yablonsky, P. K., Kolodkin, N. I., Zakharova, E. T., and Vasilyev, V. B. (2007) Interaction of ceruloplasmin, lactoferrin, and myeloperoxidase, Biochemistry (Moscow), 72, 409–415.
Sokolov, A. V., Kostevich, V. A., Romanico, D. N., Zakharova, E. T., and Vasilyev, V. B. (2012) Two-stage method for purification of ceruloplasmin based on its interaction with neomycin, Biochemistry (Moscow), 77, 631–638, doi: https://doi.org/10.1134/S0006297912060107.
Marquez, L. A., and Dunford, H. B. (1995) Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II, J. Biol. Chem., 270, 30434–30440, doi: https://doi.org/10.1074/jbc.270.51.30434.
Pfeiffer, S., Schmidt, K., and Mayer, B. (2000) Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite: implications for tyrosine modification by nitric oxide/superoxide in vivo, J. Biol. Chem., 275, 6346–6352, doi: https://doi.org/10.1074/jbc.275.9.6346.
Sokolov, A. V., Kostevich, V. A., Varfolomeeva, E. Y., Grigorieva, D. V., Gorudko, I. V., Kozlov, S. O., Kudryavtsev, I. V., Mikhalchik, E. V., Filatov, M. V., Cherenkevich, S. N., Panasenko, O. M., Arnhold, J., and Vasilyev, V. B. (2018) Capacity of ceruloplasmin to scavenge products of the respiratory burst of neutrophils is not altered by the products of reactions catalyzed by myeloperoxidase, Biochem. Cell Biol., 96, 457–467, doi: https://doi.org/10.1139/bcb-2017-0277.
Panasenko, O. M., Chekanov, A. V., Vlasova, I. I., Sokolov, A. V., Ageeva, K. V., Pulina, M. O., Cherkalina, O. S., and Vasil’ev, V. B. (2008) Influence of ceruloplasmin and lactoferrin on the chlorination activity of leukocyte myeloperoxidase assayed by chemiluminescence, Biophysics, 53, 268–272, doi: https://doi.org/10.1134/S0006350908040052.
Green, P. S., Mendez, A. J., Jacob, J. S., Crowley, J. R., Growdon, W., Hyman, B. T., and Heinecke, J. W. (2004) Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease, J. Neurochem., 90, 724–733, doi: https://doi.org/10.1111/j.1471-4159.2004.02527.x.
Malle, E., Buch, T., and Grone, H.-J. (2003) Myeloperoxidase in kidney disease, Kidney Int., 64, 1956–1967, doi: https://doi.org/10.1046/j.1523-1755.2003.00336.x.
Aouffen, M., Paquin, J., Furtos, A., Waldron, K. C., and Mateescu, M.-A. (2004) Oxidative aggregation of ceruloplasmin induced by hydrogen peroxide is prevented by pyruvate, Free Radic. Res., 38, 19–26.
Samygina, V. R., Sokolov, A. V., Bourenkov, G., Petoukhov, M. V., Pulina, M. O., Zakharova, E. T., Vasilyev, V. B., Bartunik, H., and Svergun, D. I. (2013) Ceruloplasmin: macromolecular assemblies with iron-containing acute phase proteins, PLoS One, 8, e67145, doi: https://doi.org/10.1371/journal.pone.0067145.
Kapralov, A., Vlasova, I. I., Feng, W., Maeda, A., Walson, K., Tyurin, V. A., Huang, Z., Aneja, R. K., Carcillo, J., Bayir, H., and Kagan, V. E. (2009) Peroxidase activity of hemoglobin–haptoglobin complexes. Covalent aggreation and oxidative stress in plasma and macrophages, J. Biol. Chem., 284, 30395–30407, doi: https://doi.org/10.1074/jbc.M109.045567.
Anraku, M., Yamasaki, K., Maruyama, T., Kragh-Hansen, U., and Otagiri, M. (2001) Effect of oxidative stress on the structure and function of human serum albumin, Pharm. Res., 18, 632–639.
Hawkins, C. L., Pattison, D. I., and Davies, M. J. (2003) Hypochlorite-induced oxidation of amino acids, peptides and proteins, Amino Acids, 25, 259–274, doi: https://doi.org/10.1007/s00726-003-0016-x.
Potsch, S., Lendzian, F., Ingemarson, R., Hornberg, A., Thelander, L., Lubitz, W., Lassmann, G., and Graslund, A. (1999) The iron–oxygen reconstitution reaction in protein R2-Tyr177 mutants of mouse ribonucleotide reductase: EPR and electron nuclear double resonance studies on a new transient tryptophan radical, J. Biol. Chem., 274, 17696–17704, doi: https://doi.org/10.1074/jbc.274.25.17696.
Carvalho, L. C., Estevao, M. S., Ferreira, L. M., Fernandes, E., and Marques, M. M. B. (2010) A new insight on the hypochlorous acid scavenging mechanism of tryptamine and tryptophan derivatives, Bioorg. Med. Chem. Lett., 20, 6475–6478, doi: https://doi.org/10.1016/j.bmcl.2010.09.067.
Polimova, A. M., Vladimirova, G. A., Proskurnina, E. V., and Vladimirov, Y. A. (2011) Aromatic amino acid oxidation products as antioxidants, Biophysics, 56, 585–589, doi: https://doi.org/10.1134/S000635091104021X.
Carroll, L., Pattison, D. I., Davies, J. B., Anderson, R. F., Lopez-Alarcon, C., and Davies, M. J. (2018) Superoxide radicals react with peptide-derived tryptophan radicals with very high rate constants to give hydroperoxides as major products, Free Radic. Biol. Med., 118, 126–136, doi: https://doi.org/10.1016/j.freeradbiomed.2018.02.033.
Ogasawara, Y., Namai, T., Togawa, T., and Ishii, K. (2006) Formation of albumin dimers induced by exposure to peroxides in human plasma: a possible biomarker for oxidative stress, Biochem. Biophys. Res. Commun., 340, 353–358, doi: https://doi.org/10.1016/j.bbrc.2005.11.183.
Annibal, A., Colombo, G., Milzani, A., Dalle-Donne, I., Fedorova, M., and Hoffmann, R. (2016) Identification of dityrosine cross-linked sites in oxidized human serum albumin, J. Chromatogr. B Anal. Technol. Biomed. Life Sci., 1019, 147–155, doi: https://doi.org/10.1016/j.jchromb.2015.12.022.
Colombo, G., Clerici, M., Giustarini, D., Portinaro, N., Badalamenti, S., Rossi, R., Milzani, A., and Dalle-Donne, I. (2015) A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation, Biochim. Biophys. Acta Gen. Subj., 1850, 1–12, doi: https://doi.org/10.1016/j.bbagen.2014.09.024.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2019, Vol. 84, No. 6, pp. 836-848.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM19-048, May 13, 2019.
Rights and permissions
About this article
Cite this article
Vlasova, I.I., Sokolov, A.V., Kostevich, V.A. et al. Myeloperoxidase-Induced Oxidation of Albumin and Ceruloplasmin: Role of Tyrosines. Biochemistry Moscow 84, 652–662 (2019). https://doi.org/10.1134/S0006297919060087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297919060087