Abstract
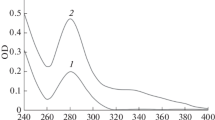
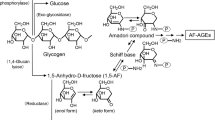
Advanced glycation end-products (AGEs) have been found to be critically involved in initiation or progression of diabetes secondary complications (nephropathy, retinopathy, neuropathy, and angiopathy). Various hyper-glycating carbonyl compounds such as 3-deoxyglucosone (3-DG) are produced in pathophysiological conditions that form AGEs in high quantity both in vivo and in vitro. In the first stage of this study, we glycated histone H2A protein by 3-DG, and the results showed the formation of various intermediates and AGEs as well as structural changes in the protein. In the second stage, we studied the immunogenicity of native and 3-DG-glycated H2A protein in female rabbits. The modified H2A was highly immunogenic, eliciting high titer immunogen-specific antibodies, while the unmodified form was almost nonimmunogenic. Antibodies against standard carboxymethyllysine (CML) and pentosidine were detected in the immunized female rabbits, which demonstrates the immunogenic nature of AGEs (CML and pentosidine) as well. The results show both structural perturbation and AGEs have the capacity of triggering the immune system due to the generation of neoepitopes that render the molecule immunogenic. This study shows the presence of autoantibodies against 3-DG-modified H2A, CML, and pentosidine in the sera of type 2 diabetes patients having secondary complications. Autoantibodies against damaged H2A and AGEs may be significant in the assessment of initiation/progression of secondary complications in type 2 diabetes mellitus patients or may be used as a marker for early detection of secondary complications in diabetes.
Similar content being viewed by others
Abbreviations
- AGEs:
-
advanced glycation end-products
- CML:
-
carboxymethyllysine
- 3-DG:
-
3-deoxyglucosone
References
Singh, R., Barden, A., Mori, T., and Beilin, L. (2001) Advanced glycation endproducts: a review, Diabetologia, 44, 129–146.
Njoroge, F. G., and Monnier, V. M. (1989) The chemistry of the Maillard reaction under physiological conditions: a review, Prog. Clin. Biol. Res., 304, 85–107.
Ahmad, S., Moinuddin, Dixit, K., Shahab, U., Alam, K., and Ali, A. (2011) Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+, Biochem. Biophys. Res. Commun., 407, 568–574.
Brownlee, M. (1992) Glycation products and pathogenesis of diabetic complications, Diabetes Care, 15, 1835–1843.
Bucala, R., Cerami, A., and Vlassara, H. (1995) Advanced glycosylation end products in diabetic complications, Diabetes Rev., 3, 258–268.
Chappey, O., Dosquet, C., Wautier, M. P., and Wautier, J. L. (1997) Advanced glycation endproducts, oxidant stress and vascular lesions, Eur. J. Clin. Invest., 27, 97–108.
Uribarri, J., Cai, W., Peppa, M., Goodman, S., Ferucci, L., Striker, G., and Vlassara, H. (2007) Circulating glycotoxins and dietary advanced glycation end products: two links to inflammatory response, oxidative stress and aging, J. Gerontol. A Biol. Sci. Med. Sci., 62, 427–433.
Park, L., Raman, K. G., Lee, K. J., Lu, Y., Ferran, L. J., Jr., Chow, W. S., Stern, D., and Schmidt, A. M. (1998) Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end products, Nat. Med., 4, 1025–1031.
McLellan, A. C., Thornalley, P. J., Benn, J., and Sonksen, P. H. (1994) Glyoxalase system in clinical diabetes mellitus and correlation with diabetic complications, Clin. Sci., 87, 21–29.
Oya, T., Hattori, N., Mizuno, Y., Miyata, S., Maeda, S., Osawa, T., and Uchida, K. (1999) Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxalarginine adducts, J. Biol. Chem., 274, 18492–18502.
Frye, E. B., Degenhardt, T. P., Thorpe, S. P., and Baynes, J. B. (1998) Role of the Maillard reaction in aging of tissue proteins. Advanced glycation end productdependent increase in imidazolium crosslinks in human lens proteins, J. Biol. Chem., 273, 18714–18719.
Thornalley, P. J., Langborg, A., and Minhas, H. S. (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose, Biochem. J., 344, 109–116.
Choudhary, D., Chandra, D., and Kale, R. K. (1997) Influence of methylglyoxal on antioxidant enzymes and oxidative damage, Toxicol. Lett., 93, 141–152.
Ashraf, J. M., Arif, B., Dixit, K., Moinuddin, and Alam, K. (2012) Physicochemical analysis of structural changes in DNA modified with glucose, Int. J. Biol. Macromol., 51, 604–611.
Festa, A., Schmolzer, B., Schernthaner, G., and Menzel, E. J. (1998) Differential expression of receptors for advanced glycation endproducts on monocytes in patients with IDDM, Diabetologia, 41, 674–680.
Wolffe, A. (1998) Chromatin. Structure and Function, Academic Press, San Diego, p. 447.
Liebich, H. M., Gesele, E., Wirth, C., Woll, J., Jobst, K., and Lakatos, A. (1992) Nonenzymatic glycation of histones, Biol. Mass Spectrom., 22, 121–123.
Gugliucci, A. (1994) Advanced glycation of rat liver histone octamers: an in vitro study, Biochem. Biophys. Res. Commun., 203, 588–593.
Cervantes-Laurean, D., Jacobson, E. L., and Jacobson, M. K. (1996) Glycation and glycoxidation of histones by ADP-ribose, J. Biol. Chem., 271, 10461–10469.
Gugliucci, A., and Bendayan, M. (1995) Histones from diabetic rats contain increased levels of advanced glycation end products, Biochem. Biophys. Res. Commun., 212, 56–62.
Bojanovic, J. J., Jevtovic, A. D., Pantic, V. S., Dugandzic, S. M., and Jovanovic, D. S. (1970) Thymus nucleoproteins. Thymus histones in young and adult rats, Gerontologia (Basel), 16, 304–312.
Turk, Z., Ljubica, S., Turk, N., and Benko, B. (2001) Detection of autoantibodies against advanced glycation end products and AGE-immune complexes in serum of patients with diabetes mellitus, Clin. Chim. Acta, 303, 105–115.
Makino, H., Shikata, K., Hironaka, K., Kushiro, M., Yamasaki, Y., Sugimoto, H., Ota, Z., Araki, N., and Horiuchi, S. (1995) Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy, Kid. Int., 48, 517–526.
Ansari, N. A., Moinuddin, Alam, K., and Ali, A. (2009) Preferential recognition of Amadoririch lysine residues by serum antibodies in diabetes mellitus: role of protein glycation in the disease process, Hum. Immunol., 70, 417–424.
Ansari, N. A., and Dash, D. (2013) Biochemical studies on methylglyoxal mediated glycated histones: implications for presence of serum antibodies against the glycated histones in patients with type 1 diabetes mellitus, ISRN Biochem., 198065.
Ashraf, J. M., Ahmad, S., Rabbani, G., Jan, A. T., Lee, E. J., Khan, R. H., and Choi, I. (2014) Physicochemical analysis of structural alteration and advanced glycation end products generation during glycation of H2A histone by 3-deoxyglucosone, IUBMB Life, 66, 686–693.
Ashraf, J. M., Arfat, M. Y., Arif, Z., Ahmad, J., Moinuddin, and Alam, K. (2015) A clinical correlation of anti-DNA-AGE autoantibodies in type 2 diabetes mellitus with disease duration, Cell Immunol., 293, 74–79.
Shahab, U., Moinuddin, Ahmad, S., Dixit, K., and Ali, A. (2013) Genotoxic effect of N-hydroxy-4-acetylaminobiphenyl on human DNA: implications in bladder cancer, PLoS One, 8, e53205.
Moinuddin, Dixit, K., Ahmad, S., Shahab, U., Alam, K., and Ali, A. (2014) Human DNA damage by the synergistic action of 4-aminobiphenyl and nitric oxide: an immunochemical study, Environ. Toxicol., 29, 568–576.
Alam, K., Moinuddin, and Jabeen, S. (2007) Immunogenicity of mitochondrial DNA modified by hydroxyl radical, Cell Immunol., 247, 12–17.
Ashraf, J. M., Rabbani, G., Ahmad, S., Hasan, Q., Khan, R. H., Alam, K., and Choi, I. (2015) Glycation of H1 histone by 3-deoxyglucosone: effects on protein structure and generation of different advanced glycation end products, PLoS One, 10, e0130630.
Ashraf, J. M., Ahmad, S., Rabbani, G., Hasan, Q., Jan, A. T., Lee, E. J., and Choi, I. (2015) 3-Deoxyglucosone: a potential glycating agent accountable for structural alteration in H3 histone protein through generation of different AGEs, PLoS One, 10, e0116804.
Arfat, M. Y., Ashraf, J. M., Arif, Z., Moinuddin, and Alam, K. (2014) Fine characterization of glucosylated human IgG by biochemical and biophysical methods, Int. J. Biol. Macromol., 69, 408–415.
Akhter, F., Khan, M. S., Singh, S., and Ahmad, S. (2014) An immunohistochemical analysis to validate the rationale behind the enhanced immunogenicity of D-ribosylated lowdensity lipoprotein, PLoS One, 9, e113144.
Turk, Z., Ljubica, S., Turk, N., and Benko, B. (2001) Detection of autoantibodies against advanced glycation end products and AGE-immune complexes in serum of patients with diabetes mellitus, Clin. Chim. Acta, 303, 105–115.
Mustafa, I., Ahmad, S., Dixit, K., Moinuddin, Ahmad, J., and Ali, A. (2012) Glycated human DNA is a preferred antigen for anti-DNA antibodies in diabetes mellitus patients, Diabetes Res. Clin. Prac., 95, 98–104.
Nagai, R., Fujiwara, Y., Mera, K., Yamagata, K., Sakashita, N., and Takeya, M. (2008) Immunochemical detection of N epsilon-(carboxyethyl)lysine using a specific antibody, J. Immunol. Methods, 332, 112–120.
Ikeda, K., Higashi, T., Sano, H., Jinnouchi, Y., Yoshida, M., Araki, T., Ueda, S., and Horiuchi, S. (1996) Nε-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction, Biochemistry, 35, 8075–8083.
Reddy, S., Bichler, J., Wells-Knecht, K. J., Thorpe, S. R., and Baynes, J. W. (1995) Nε-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins, Biochemistry, 34, 10872–10878.
Shibayama, R., Araki, N., Nagai, R., and Horiuchi, S. (1999) Autoantibody against Nε-carboxymethyllysine: an advanced glycation end product of the Maillard reaction, Diabetes, 48, 1842–1829.
Ahmad, S., Moinuddin, Habib, S., Shahab, U., Alam, K., and Ali, A. (2014) Autoimmune response to AGE modified human DNA: implications in type 1 diabetes mellitus, J. Clin. Trans Endo, 1, 66–72.
Ahmad, S. (2014) Immunogenicity of DNA damage by free radicals and carbonyls: a probable biomarker for the autoimmune diseases, J. Immun. Res., 1, 2.
Shahab, U., Tabrez, S., Khan, M. S., Akhter, F., Khan, M. S., Saeed, M., Ahmad, K., Srivastava, A. K., and Ahmad, S. (2014) Immunogenicity of DNA-advanced glycation end product fashioned through glyoxal and arginine in the presence of Fe3+: its potential role in prompt recognition of diabetes mellitus autoantibodies, Chem. Biol. Int., 219, 229–240.
Ahmad, S., Moinuddin, Shahab, U., Khan, M. S., Habeeb, S., Alam, K., and Ali, A. (2014) Glycooxidative damage to human DNA-neoantigenic epitopes on DNA molecule could be a possible reason for autoimmune response in type 1 diabetes, Glycobiology, 24, 281–291.
Akhter, F., Khan, M. S., Abdulrahman, A. A., Faisal, M., and Ahmad, S. (2016) Antigenic role of the adaptive immune response to D-ribose glycated LDL in diabetes, atherosclerosis and diabetic atherosclerosis patients, Life Sci., 151, 139–146.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2017, Vol. 82, No. 5, pp. 776-785.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM16-333, February 6, 2017.
To whom correspondence should be addressed.
Rights and permissions
About this article
Cite this article
Ashraf, J.M., Abdullah, S.M.S., Ahmad, S. et al. Prevalence of autoantibodies against 3-DG-glycated H2A protein in type 2 diabetes. Biochemistry Moscow 82, 579–586 (2017). https://doi.org/10.1134/S0006297917050066
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917050066