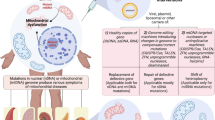
Abstract
Apart from the nucleus, the mitochondrion is the only organelle of an animal cell that contains its own genome. Mitochondrial DNA is much less in size than the nuclear one and codes for only several dozens of biological macromolecules. Nevertheless, mutations in mitochondrial genes often result in the occurrence of serious hereditary neuromuscular diseases. New mitochondrial DNA mutations and their relations to clinical symptoms are continuously reported in the scientific literature. In this review, we summarize existing data about such mutations, and also about contemporary gene therapy approaches that have been developed for their suppression.
Similar content being viewed by others
Abbreviations
- mtDNA:
-
mitochondrial DNA
References
Naviaux, R. K. (2004) Developing a systematic approach to the diagnosis and classification of mitochondrial disease, Mitochondrion, 4, 351–361.
Luft, R., Ikkos, D., Palmieri, G., Ernster, L., and Afzelius, B. (1962) A case of severe hypermetabolism of nonthyroid origin with a defect in the maintenance of mitochondrial respiratory control: a correlated clinical, biochemical, and morphological study, J. Clin. Invest., 41, 1776–1804.
DiMauro, S., Bonilla, E., Zeviani, M., Nakagawa, M., and DeVivo, D. C. (1985) Mitochondrial myopathies, Ann. Neurol., 17, 521–538.
Nass, S., and Nass, M. M. (1963) Intramitochondrial fibers with DNA characteristics. II. Enzymatic and other hydrolytic treatments, J. Cell Biol., 19, 613–629.
Montoya, J., Christianson, T., Levens, D., Rabinowitz, M., and Attardi, G. (1982) Identification of initiation sites for heavy-strand and light-strand transcription in human mitochondrial DNA, Proc. Natl. Acad. Sci. USA, 79, 7195–7199.
Wallace, D. C., Singh, G., Lott, M. T., Hodge, J. A., Shurr, T. G., Lezza, A. M., Elsas, L. J., 2nd, and Nikoskelainen, E. K. (1988) Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy, Science, 242, 1427–1430.
Holt, I. J., Harding, A. E., and Morgan-Hughes, J. A. (1988) Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies, Nature, 331, 717–719.
Dimauro, S. (2011) A history of mitochondrial diseases, J. Inherit. Metab. Dis., 34, 261–276.
Spelbrink, J. N. (2010) Functional organization of mammalian mitochondrial DNA in nucleoids: history, recent developments, and future challenges, IUBMB Life, 62, 19–32.
Anderson, S., Bankier, A. T., Barrell, B. G., de Bruijn, M. H., Coulson, A. R., Drouin, J., Eperon, I. C., Nierlich, D. P., Roe, B. A., Sanger, F., Schreier, P. H., Smith, A. J., Staden, R., and Young, I. G. (1981) Sequence and organization of the human mitochondrial genome, Nature, 290, 457–465.
Lightowlers, R. N., Chinnery, P. F., Turnbull, D. M., and Howell, N. (1997) Mammalian mitochondrial genetics: heredity, heteroplasmy and disease, Trends Genet., 13, 450–455.
Chinnery, P. F., Howell, N., Lightowlers, R. N., and Turnbull, D. M. (1998) Genetic counseling and prenatal diagnosis for mtDNA disease, Am. J. Hum. Genet., 63, 1908–1911.
Cummins, J. M. (2000) Fertilization and elimination of the paternal mitochondrial genome, Hum. Reprod., 15, 92–101.
Schwartz, M., and Vissing, J. (2002) Paternal inheritance of mitochondrial DNA, N. Engl. J. Med., 347, 576–580.
Wonnapinij, P., Chinnery, P. F., and Samuels, D. C. (2008) The distribution of mitochondrial DNA heteroplasmy due to random genetic drift, Am. J. Hum. Genet., 83, 582–593.
Gilkerson, R. W., and Schon, E. A. (2008) Nucleoid autonomy: an underlying mechanism of mitochondrial genetics with therapeutic potential, Commun. Integr. Biol., 1, 34–36.
Dean, N. L., Battersby, B. J., Ao, A., Gosden, R. G., Tan, S. L., Shoubridge, E. A., and Molnar, M. J. (2003) Prospect of pre-implantation genetic diagnosis for heritable mitochondrial DNA diseases, Mol. Hum. Reprod., 9, 631–638.
De Laat, P., Koene, S., Heuvel, L. P., Rodenburg, R. J., Janssen, M. C., and Smeitink, J. A. (2013) Inheritance of the m.3243A>G mutation, JIMD Rep., 8, 47–50.
Shoubridge, E. A., and Wai, T. (2007) Mitochondrial DNA and the mammalian oocyte, Curr. Top Dev. Biol., 77, 87–111.
Calvo, S. E., and Mootha, V. K. (2010) The mitochondrial proteome and human disease, Annu. Rev. Genom. Hum. Genet., 11, 25–44.
Harbauer, A. B., Zahedi, R. P., Sickmann, A., Pfanner, N., and Meisinger, C. (2014) The protein import machinery of mitochondria — a regulatory hub in metabolism, stress, and disease, Cell Metab., 4, 357–372.
Wang, J., Schmitt, E. S., Landsverk, M. L., Zhang, V. W., Li, F. Y., Graham, B. H., Craigen, W. J., and Wong, L. J. (2012) An integrated approach for classifying mitochondrial DNA variants: one clinical diagnostic laboratory’s experience, Genet. Med., 14, 620–626.
DiMauro, S., and Schon, E. A. (2001) Mitochondrial DNA mutations in human disease, Am. J. Med. Genet., 106, 18–26.
Bosley, T. M., and Abu-Amero, K. K. (2010) Assessing mitochondrial DNA nucleotide changes in spontaneous optic neuropathies, Ophthalm. Genet., 31, 163–172.
Schiff, M., Benit, P., Jacobs, H. T., Vockley, J., and Rustin, P. (2012) Therapies in inborn errors of oxidative metabolism, Trends Endocrinol. Metab., 23, 488–495.
Manfredi, G., Fu, J., Ojaimi, J., Sadlock, J. E., Kwong, J. Q., Guy, J., and Schon, E. A. (2002) Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus, Nature Genet., 30, 394–399.
Kaltimbacher, V., Bonnet, C., Lecoeuvre, G., Forster, V., Sahel, J. A., and Corral-Debrinski, M. (2006) mRNA localization to the mitochondrial surface allows the efficient translocation inside the organelle of a nuclear recoded ATP6 protein, RNA, 12, 1408–1417.
Bonnet, C., Kaltimbacher, V., Ellouze, S., Augustin, S., Benit, P., Forster, V., Rustin, P., Sahel, J. A., and Corral-Debrinski, M. (2007) Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v-subunits, Rejuven. Res., 10, 127–144.
Ojaimi, J., Pan, J., Santra, S., Snell, W. J., and Schon, E. A. (2002) An algal nucleus-encoded subunit of mitochondrial ATP synthase rescues a defect in the analogous human mitochondrial-encoded subunit, Mol. Biol. Cell, 13, 3836–3844.
Tanaka, M., Borgeld, H. J., Zhang, J., Muramatsu, S., Gong, J. S., Yoneda, M., Maruyama, W., Naoi, M., Ibi, T., Sahashi, K., Shamoto, M., Fuku, N., Kurata, M., Yamada, Y., Nishizawa, K., Akao, Y., Ohishi, N., Miyabayashi, S., Umemoto, H., Muramatsu, T., Furukawa, K., Kikuchi, A., Nakano, I., Ozawa, K., and Yagi, K. (2002) Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria, J. Biomed. Sci., 9, 534–541.
Alexeyev, M. F., Venediktova, N., Pastukh, V., Shokolenko, I., Bonilla, G., and Wilson, G. L. (2008) Selective elimination of mutant mitochondrial genomes as therapeutic strategy for the treatment of NARP and MILS syndromes, Gene Ther., 15, 516–523.
Bacman, S. R., Williams, S. L., Garcia, S., and Moraes, C. T. (2010) Organ-specific shifts in mtDNA heteroplasmy following systemic delivery of a mitochondria-targeted restriction endonuclease, Gene Ther., 17, 713–720.
Minczuk, M., Papworth, M. A., Kolasinska, P., Murphy, M. P., and Klug, A. (2006) Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase, Proc. Natl. Acad. Sci. USA, 103, 19689–19694.
Gammage, P. A., Rorbach, J., Vincent, A. I., Rebar, E. J., and Minczuk, M. (2014) Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations, EMBO Mol. Med., 6, 458–466.
Guy, J., Qi, X., Pallotti, F., Schon, E. A., Manfredi, G., Carelli, V., Martinuzzi, A., Hauswirth, W. W., and Lewin, A. S. (2002) Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy, Ann. Neurol., 52, 534–542.
Yu, H., Koilkonda, R. D., Chou, T. H., Porciatti, V., Ozdemir, S. S., Chiodo, V., Boye, S. L., Boye, S. E., Hauswirth, W. W., Lewin, A. S., and Guy, J. (2012) Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model, Proc. Natl. Acad. Sci. USA, 109, 1238–1247.
Park, J. S., Li, Y. F., and Bai, Y. (2007) Yeast NDI1 improves oxidative phosphorylation capacity and increases protection against oxidative stress and cell death in cells carrying a Leber’s hereditary optic neuropathy mutation, Biochim. Biophys. Acta, 1772, 533–542.
Bonnet, C., Augustin, S., Ellouze, S., Benit, P., Bouaita, A., Rustin, P., Sahel, J. A., and Corral-Debrinski, M. (2008) The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes, Biochim. Biophys. Acta, 1783, 1707–1717.
Bacman, S. R., Williams, S. L., Pinto, M., Peralta, S., and Moraes, C. T. (2013) Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs, Nature Med., 19, 1111–1113.
Kolesnikova, O. A., Entelis, N. S., Jacquin-Becker, C., Goltzene, F., Chrzanowska-Lightowlers, Z. M., Lightowlers, R. N., Martin, R. P., and Tarassov, I. (2004) Nuclear DNA-encoded tRNAs targeted into mitochondria can rescue a mitochondrial DNA mutation associated with the MERRF syndrome in cultured human cells, Hum. Mol. Genet., 13, 2519–2534.
Kolesnikova, O. A., Entelis, N. S., Mireau, H., Fox, T. D., Martin, R. P., and Tarassov, I. A. (2000) Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm, Science, 289, 1931–1933.
Tarassov, I., Kamenski, P., Kolesnikova, O., Karicheva, O., Martin, R. P., Krasheninnikov, I. A., and Entelis, N. (2007) Import of nuclear DNA-encoded RNAs into mitochondria and mitochondrial translation, Cell Cycle, 6, 2473–2477.
Wang, G., Shimada, E., Zhang, J., Hong, J. S., Smith, G. M., Teitell, M. A., and Koehler, C. M. (2012) Correcting human mitochondrial mutations with targeted RNA import, Proc. Natl. Acad. Sci. USA, 109, 4840–4845.
Ling, J., Yadavalli, S. S., and Ibba, M. (2007) Phenylalanyl-tRNA synthetase editing defects result in efficient mistranslation of phenylalanine codons as tyrosine, RNA, 13, 1881–1886.
Park, H., Davidson, E., and King, M. P. (2008) Overexpressed mitochondrial leucyl-tRNA synthetase suppresses the A3243G mutation in the mitochondrial tRNA (Leu (UUR)) gene, RNA, 14, 2407–2416.
Karicheva, O. Z., Kolesnikova, O. A., Schirtz, T., Vysokikh, M. Y., Mager-Heckel, A. M., Lombes, A., Boucheham, A., Krasheninnikov, I. A., Martin, R. P., Entelis, N., and Tarassov, I. (2011) Correction of the consequences of mitochondrial 3243A>G mutation in the MT-TL1 gene causing the MELAS syndrome by tRNA import into mitochondria, Nucleic Acids Res., 39, 8173–8186.
Comte, C., Tonin, Y., Heckel-Mager, A. M., Boucheham, A., Smirnov, A., Aure, K., Lombes, A., Martin, R. P., Entelis, N., and Tarassov, I. (2013) Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns-Sayre Syndrome, Nucleic Acids Res., 41, 418–433.
Schon, E. A., Santra, S., Pallotti, F., and Girvin, M. E. (2001) Pathogenesis of primary defects in mitochondrial ATP synthesis, Sem. Cell Dev. Biol., 12, 441–448.
Solaini, G., Harris, D. A., Lenaz, G., Sgarbi, G., and Baracca, A. (2008) The study of the pathogenic mechanism of mitochondrial diseases provides information on basic bioenergetics, Biochim. Biophys. Acta, 1777, 941–945.
Holt, I. J., Harding, A. E., Petty, R. K., and Morgan-Hughes, J. A. (1990) A new mitochondrial disease associated with mitochondrial DNA heteroplasmy, Am. J. Hum. Genet., 46, 428–433.
Tatuch, Y., Christodoulou, J., Feigenbaum, A., Clarke, J. T., Wherret, J., Smith, C., Rudd, N., Petrova-Benedict, R., and Robinson, B. H. (1992) Heteroplasmic mtDNA mutation (T-G) at 8993 can cause Leigh disease when the percentage of abnormal mtDNA is high, Am. J. Hum. Genet., 50, 852–858.
Bacman, S. R., Williams, S. L., Duan, D., and Moraes, C. T. (2012) Manipulation of mtDNA heteroplasmy in all striated muscles of newborn mice by AAV9-mediated delivery of a mitochondria-targeted restriction endonuclease, Gene Ther., 19, 1101–1106.
Sadun, A. A., La Morgia, C., and Carelli, V. (2011) Leber’s hereditary optic neuropathy, Curr. Treat. Options Neurol., 13, 109–117.
Huoponen, K., Vilkki, J., Aula, P., Nikoskelainen, E. K., and Savontaus, M. L. (1991) A new mtDNA mutation associated with Leber hereditary optic neuroretinopathy, Am. J. Hum. Genet., 48, 1147–1153.
Jun, A. S., Trounce, I. A., Brown, M. D., Shoffner, J. M., and Wallace, D. C. (1996) Use of trans-mitochondrial cybrids to assign a complex I defect to the mitochondrial DNA-encoded NADH dehydrogenase subunit 6 gene mutation at nucleotide pair 14,459 that causes Leber hereditary optic neuropathy and dystonia, Mol. Cell Biol., 16, 771–777.
Cermak, T., Doyle, E. L., Christian, M., Wang, L., Zhang, Y., Schmidt, C., Baller, J. A., Somia, N. V., Bogdanove, A. J., and Voytas, D. F. (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting, Nucleic Acids Res., 39, e82.
Silvestri, G., Ciafaloni, E., Santorelli, F. M., Shanske, S., Servidei, S., Graf, W. D., Sumi, M., and DiMauro, S. (1993) Clinical features associated with the A->G transition at nucleotide 8344 of mtDNA (“MERRF mutation”), Neurology, 43, 1200–1206.
Tonin, Y., Heckel, A. M., Dovydenko, I., Meschaninova, M., Comte, C., Venyaminova, A., Pyshnyi, D., Tarassov, I., and Entelis, N. (2014) Characterization of chemically modified oligonucleotides targeting a pathogenic mutation in human mitochondrial DNA, Biochimie, 100, 192–199.
Mancuso, M., Filosto, M., Mootha, V. K., Rocchi, A., Pistolesi, S., Murri, L., DiMauro, S., and Siciliano, G. (2004) A novel mitochondrial tRNAPhe mutation causes MERRF syndrome, Neurology, 62, 2119–2121.
Suzuki, T., Nagao, A., and Suzuki, T. (2011) Human mitochondrial tRNAs: biogenesis, function, structural aspects, and diseases, Annu. Rev. Genet., 45, 299–329.
Schon, E. A., Rizzuto, R., Moraes, C. T., Nakase, H., Zeviani, M., and DiMauro, S. (1989) A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA, Science, 244, 346–349.
Maceluch, J. A., and Niedziela, M. (2006) The clinical diagnosis and molecular genetics of Kearns-Sayre syndrome: a complex mitochondrial encephalomyopathy, Pediatr. Endocrinol. Rev., 4, 117–137.
Corral-Debrinski, M., Horton, T., Lott, M. T., Shoffner, J. M., Beal, M. F., and Wallace, D. C. (1992) Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age, Nature Genet., 2, 324–329.
Kolesnikova, O., Kazakova, H., Comte, C., Steinberg, S., Kamenski, P., Martin, R. P., Tarassov, I., and Entelis, N. (2010) Selection of RNA aptamers imported into yeast and human mitochondria, RNA, 16, 926–941.
Dimauro, S. (2004) Mitochondrial medicine, Biochim. Biophys. Acta, 1659, 107–114.
Pfeffer, G., Horvath, R., Klopstock, T., Mootha, V. K., Suomalainen, A., Koene, S., Hirano, M., Zeviani, M., Bindoff, L. A., Yu-Wai-Man, P., Hanna, M., Carelli, V., McFarland, R., Majamaa, K., Turnbull, D. M., Smeitink, J., and Chinnery, P. F. (2013) New treatments for mitochondrial disease — no time to drop our standards, Nature Rev. Neurol., 9, 474–481.
Footitt, E. J., Sinha, M. D., Raiman, J. A., Dhawan, A., Moganasundram, S., and Champion, M. P. (2008) Mitochondrial disorders and general anaesthesia: a case series and review, Br. J. Anaesth., 100, 436–441.
Tachibana, M., Amato, P., Sparman, M., Woodward, J., Sanchis, D. M., Ma, H., Gutierrez, N. M., Tippner-Hedges, R., Kang, E., Lee, H. S., Ramsey, C., Masterson, K., Battaglia, D., Lee, D., Wu, D., Jensen, J., Patton, P., Gokhale, S., Stouffer, R., and Mitalipov, S. (2013) Towards germline gene therapy of inherited mitochondrial diseases, Nature, 493, 627–631.
Bredenoord, A. L., Dondorp, W., Pennings, G., and De Wert, G. (2011) Ethics of modifying the mitochondrial genome, J. Med. Ethics, 37, 97–100.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Original Russian Text © M. V. Patrushev, P. A. Kamenski, I. O. Mazunin, 2014, published in Biokhimiya, 2014, Vol. 79, No. 11, pp. 1417–1428.
Rights and permissions
About this article
Cite this article
Patrushev, M.V., Kamenski, P.A. & Mazunin, I.O. Mutations in mitochondrial DNA and approaches for their correction. Biochemistry Moscow 79, 1151–1160 (2014). https://doi.org/10.1134/S0006297914110029
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297914110029