Abstract
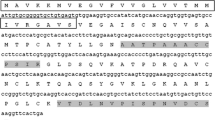
Homologs of vitamin K epoxide reductase (VKOR) exist widely in plants. However, only VKOR of Arabidopsis thaliana has been the subject of many studies to date. In the present study, the coding region of a VKOR from Solanum lyco-persicum (JF951971 in GenBank) was cloned; it contained a membrane domain (VKOR domain) and an additional soluble thioredoxin-like (Trx-like) domain. Bioinformatic analysis showed that the first 47 amino acids in the N-terminus should act as a transit peptide targeting the protein to the chloroplast. Western blot demonstrated that the protein is localized in thylakoid membrane with the Trx-like domain facing the lumen. Modeling of three-dimensional structure showed that SlVKOR has a similar conformation with Arabidopsis and cyanobacterial VKORs, with five transmembrane segments in the VKOR domain and a typical Trx-like domain in the lumen. Functional assay showed that the full-length of SlVKOR with Trx-like domain without the transit peptide could catalyze the formation of disulfide bonds. Similar transit peptides at the N-terminus commonly exist in plant VKORs, most of them targeting to chloroplast according to prediction. Comparison of sequences and structures from different plants indicated that all plant VKORs possess two domains, a transmembrane VKOR domain and a soluble Trx-like domain, each having four conservative cysteines. The cysteines were predicted to be related to the function of catalyzing the formation of disulfide bonds.
Similar content being viewed by others
References
Cain, D., Hutson, S. M., and Wallin, R. (1997) J. Biol. Chem., 272, 29068–29075.
Li, W., Schulman, S., Dutton, R. J., Boyd, D., Beckwith, J., and Rapoport, T. A. (2010) Nature, 463, 507–512.
Singh, A. K., Bhattacharyya-Pakrasi, M., and Pakrasi, H. B. (2008) J. Biol. Chem., 283, 15762–15770.
Goodstadt, L., and Ponting, C. P. (2004) Trends Biochem. Sci., 29, 289–292.
Furt, F., Oostende, C., Widhalm, J. R., Dale, M. A., Wertz, J., and Basset, G. J. (2010) Plant J., 64, 38–46.
Feng, W. K., Wang, L., Lu, Y., and Wang, X. Y. (2011) FEBS J., 278, 3419–3430.
Karamoko, M., Cline, S., Redding, K., Ruiz, N., and Hamel, P. P. (2011) Plant Cell, 23, 4462–4475.
Lu, Y., Wang, H. R., Li, H., Cui, H. R., Feng, Y. G., and Wang, X. Y. (2013) Plant Cell Rep., 32, 1427–1440.
Guzman, L. M., Barondess, J. J., and Beckwith, J. (1992) J. Bacteriol., 174, 7716–7728.
Armbruster, U., Zuhlke, J., Rengstl, B., Kreller, R., Makarenko, E., Ruhle, T., Schunemann, D., Jahns, P., Weisshaar, B., Nickelsen, J., and Leister, D. (2010) Plant Cell, 22, 3439–3460.
Jander, G., Martin, N. L., and Beckwith, J. (1994) EMBO J., 13, 5121–5127.
Tian, H., Boyd, D., and Beckwith, J. (2000) Proc. Natl. Acad. Sci. USA, 97, 4730–4735.
Arnold, K., Bordoli, L., Kopp, J., and Schwede, T. (2006) Bioinformatics, 22, 195–201.
Schwede, T., Kopp, J., Guex, N., and Peitsch, M. C. (2003) Nucleic Acids Res., 31, 3381–3385.
Guex, N., and Peitsch, M. C. (1997) Electrophoresis, 18, 2714–2723.
Bardwell, J. C., McGovern, K., and Beckwith, J. (1991) Cell, 67, 581–589.
Bardwell, J. C., Lee, J. O., Jander, G., Martin, N., Belin, D., and Beckwith, J. (1993) Proc. Natl. Acad. Sci. USA, 90, 1038–1042.
Dailey, F. E., and Berg, H. C. (1993) J. Bacteriol., 175, 3236–3239.
Kadokura, H., Bader, M., Tian, H., Bardwell, J. C., and Beckwith, J. (2000) Proc. Natl. Acad. Sci. USA, 97, 10884–10889.
Goyer, A., Haslekas, C., Miginiac-Maslow, M., Klein, U., Le Marechal, P., Jacquot, J. P., and Decottignies, P. (2002) Eur. J. Biochem., 269, 272–282.
Martin, J. L. (1995) Structure, 3, 245–250.
Rost, S., Fregin, A., Ivaskevicius, V., Conzelmann, E., Hortnagel, K., Pelz, H. J., Lappegard, K., Seifried, E., Scharrer, I., Tuddenham, E. G., Muller, C. I., Strom, T. M., and Oldenburg, J. (2004) Nature, 427, 537–541.
Oldenburg, J., Bevans, C. G., Muller, C. R., and Watzka, M. (2006) Antioxid. Redox Signal, 8, 347–353.
Kadokura, H., and Beckwith, J. (2010) Antioxid. Redox Signal, 13, 1231–1246.
Kadokura, H., and Beckwith, J. (2002) EMBO J., 21, 2354–2363.
Eser, M., Masip, L., Kadokura, H., Georgiou, G., and Beckwith, J. (2009) Proc. Natl. Acad. Sci. USA, 106, 1572–1577.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Biokhimiya, 2014, Vol. 79, No. 5, pp. 560–571.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM13-345, February 23, 2014.
These authors contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wan, CM., Yang, XJ., Du, JJ. et al. Identification and characterization of SlVKOR, a disulfide bond formation protein from Solanum lycopersicum, and bioinformatic analysis of plant VKORs. Biochemistry Moscow 79, 440–449 (2014). https://doi.org/10.1134/S0006297914050083
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297914050083