Abstract
The interaction of chitosan with 3-(chloromethyl)-[1,2,4]selendiazole[4,5-a]pyridin-4 bromide results in water-soluble, selenium-containing, cationic chitosan derivatives. Derivatives of chitosan with degrees of substitution of 0.15, 0.45, and 0.65 were obtained. These derivatives are characterized by a pronounced in vitro antibacterial activity against Staphylococcus aureus and Escherichia coli, and the antibacterial activity of the derivatives increases with an increase in their degree of substitution. The antibacterial activity of the highly substituted derivative is comparable to that of the conventional antibiotics ampicillin and gentamicin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Due to the steady increase in the resistance of pathogens of nosocomial and community-acquired infections to antimicrobial drugs, the development and synthesis of new, effective antibacterial drugs is a high-priority task of modern pharmacology. Chitosan is favorable among the various compounds with antibacterial activity due to its biocompatibility, hypoallergenicity, biodegradability, and lack of toxicity.
Chitosan is a linear, natural polymer composed of glucosamine units that alternate with N-acetylglucosamine units. Chitosan derivatives are often characterized by pronounced antibacterial activity [1]. It is known that chitosan derivatives have several mechanisms of antibacterial action at once, which are, in essence, universal physicochemical mechanisms: the electrostatic interaction of a polycation with a negatively charged surface of a bacterial cell, the chelation and binding of ions and nutrients important for a microbial cell, etc. Due to the complex antibacterial effect of chitosan derivatives, the manifestation of bacterial resistance to these polymers in the near future is unlikely, if not impossible [2].
In the complex mechanism of the antibacterial action of chitosan, a key point is the interaction of the chitosan polycation with the negatively charged surface of the bacterial cell. The chitosan polycation forms due to the basic properties of the primary amino groups of the chitosan macromolecule. The electrostatic interaction of such a polycation with a bacterial cell leads to at least two unfavorable consequences for the cell: a sharp change in membrane permeability, which causes an internal osmotic imbalance and, therefore, inhibits microorganism growth. In addition, peptidoglycans are hydrolytically cleaved in the wall of the microorganism, which leads to leakage of the intracellular electrolytes, such as potassium ions, as well as important components of organic nature, such as proteins, nucleic acids, glucose, lactate dehydrogenase, etc. [3]. These processes, which are unfavorable for the bacterial cell, ultimately lead to its death.
Characteristics of chitosan that severely limit its antibacterial effect are its poor solubility in water and low cationic density. The introduction of a cationic substituent can overcome these limitations [4–6]. Usually, chemical modification of chitosan with a substituent containing a quaternized nitrogen atom is used for these purposes.
It is also known that a number of selenium-containing heterocycles are characterized by a pronounced antibacterial effect and pharmacological activity [7]. In this regard, selenium-containing derivatives of chitosan are of undoubted interest as potential antibacterial agents; however, they have not been described in the literature until this study.
The goal of this work is to chemically modify chitosan with a selenium-containing cationic heterocyclic fragment and to study the antibacterial activity of the obtained chitosan derivatives in comparison with the initial chitosan and the antibiotics gentamicin and ampicillin against gram-positive and gram-negative bacteria (Staphylococcus aureus and Escherichia coli).
METHODOLOGY
Crab chitosan (OOO Bioprogress, Russia) with an average molecular weight of 3.6 × 104, a degree of acetylation of 0.26, a humidity of 8.8%, and 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide kindly provided by A.G. Tskhovrebov (Peoples’ Friendship University of Russia, Russia) was used in the study. The dialysis membranes (MWCO 12 000–14 000) were purchased from Orange Scientific (Braine-l’Alleud, Belgium). The nuclear magnetic resonance (NMR) spectra of 1Н was recorded on a Bruker Avance instrument (Bruker, United States) II + 400 MHz in a solution of D2O/CF3COOH 100/1 at 70°C. The integral intensity of H-1 signals from glucosamine fragments of chitosan and its derivatives was taken as 1.
Selenium-containing derivatives of chitosan were synthesized as follows: 0.1 g of chitosan was dissolved in 10 mL of 1% acetic acid (pH of 3.0), and 2.2, 6.0, or 13.5 equivalents of 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide. The reaction mixture was sealed, bubbled with argon, and stirred at 60°C for 7 h on a magnetic stirrer. The resulting polymers were precipitated with acetone, washed free of impurities from low-molecular compounds with methanol and diethyl ether, dissolved in distilled water, dialyzed against distilled water for 3 days, and then freeze-dried.
The antibacterial activity of the obtained chitosan derivatives against the strains S. aureus RCMB 010027 and E. coli RCMB 010051 (collection of microorganisms of Vitebsk State Medical University, Belarus) was studied via diffusion in agar. The commercially available antibiotics used in the comparison were ampicillin (for S. aureus) and gentamicin (for E. coli) (Aldrich, United States). The activity was determined from measurements of the diameter of the zone of inhibition (in mm). Each zone of inhibition was measured after overnight cultivation in an incubator at 37°C. The experiments were repeated at least three times [8].
RESULTS AND DISCUSSION
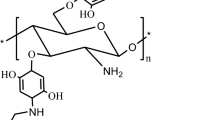
Selenium-containing derivatives of chitosan were obtained via chitosan treatment with 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide (Fig. 1). The reaction was carried out at 60°C (pH of 3) for 7 h.
Synthesis of selenium-containing derivatives of chitosan: 1, chitosan; 2, (chloromethyl)-[1, 2, 4]selenadiazole[4,5-a]pyridinium bromide; 3, N-([1, 2, 4]selenadiazole[4,5-a]pyridin-3-ylmethyl) chitosan; 4, N, O-([1, 2, 4]selenadiazole[4,5-a]pyridin-3-ylmethyl) chitosan; I, reaction with a slight excess of the reagent 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide; II, reaction with a large excess of the reagent 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide.
Variation of the excess of 3-(chloromethyl)-[1, 2, 4]selendiazole[4,5-a]pyridine-4 bromide made it possible to obtain chitosan derivatives with low (0.15), medium (0.45), and high (0.65) degrees of substitution (DS). Upon a slight excess of reagent in the synthesis of low- and medium-substituted derivatives, the reaction proceeded selectively at the amino group of chitosan with the formation N-substituted polymers (Fig. 1, reaction I). With the use of a large excess of regent (13.5-fold), highly substituted derivatives formed (DS = 0.65), while the proportion of N substitution (DSN) was 0.50, and the proportion of O substitution (DSO) was 0.15 (Fig. 1, reaction II). The structure of the obtained compounds was confirmed via NMR spectroscopy on 1N nuclei. Figure 2 shows a typical spectrum.
The antibacterial activity of the obtained derivatives was studied in vitro with the agar-diffusion method. The research results are presented in Table 2.
The data given in Table 2 indicate that the antibacterial activity of all of the obtained chitosan derivatives exceeded that of the initial chitosan. In this case, the antibacterial effect of the derivatives increased with an increase in the degree of substitution. On the one hand, this can be explained by the increase in cationic density upon an increase in the proportion of the cationic substituent in the macromolecule. On the other hand, it is possible that the introduced heterocyclic, selenium-containing substituent is itself characterized by the presence of a pronounced antibacterial effect. To test this assumption, the antibacterial activity of low molecular weight compounds b and c, which correspond to the introduce substituents, was studied (Fig. 3).
CONCLUSIONS
It has been shown that the low molecular weight compounds b and c, which are characterized by pronounced antibacterial activity against both S. aureus and E. coli, and their antibacterial activity is comparable to that of the antibiotics ampicillin and gentamicin. It should also be noted that the highly substituted selenium-containing chitosan derivative X-Se-65 was characterized by an antibacterial effect exceeding that of ampicillin and gentamicin. This fact can be explained by the symbatic effect of the introduction of the antibacterial, selenium-containing pharmacophore and polymer chain into the chitosan matrix. Apparently, this is due to the fact that the polymer chain is capable of assuming a conformation that ensures its strongest binding to the membrane of a microbial cell in comparison with a low-molecular-weight compound and, therefore, leads to a more pronounced dysfunction of the cell membrane.
Thus, as a result of the work, a highly active, antibacterial, cationic, selenium-containing chitosan derivative was obtained, and it is of undoubted interest for further in vivo research.
REFERENCES
Seidi, F., Yazdi, M.K., Jouyandeh, M., Dominic, M., Naeim, H., Nezhad, M.N., Bagheri, B., Habibzadeh, S., Zarrintaj, P., Saeb, M.R., and Mozafari, M., Int. J. Biol. Macromol., 2021, vol. 183, pp. 1818–1850.
Tien, N.D., Lyngstadaas, S.P., Mano, J.F., Blaker, J.J., and Haugen, H.J., Molecules, 2021, vol. 26, no. 9. https://doi.org/10.3390/molecules26092683
Ke, C.L., Deng, F.S., Chuang, C.Y., and Lin, C.H., Polymers, 2021, vol. 13, no. 6. https://doi.org/10.3390/polym13060904
Kritchenkov, A.S., Egorov, A.R., Dysin, A.P., Volkova, O.V., Zabodalova, L.A., Suchkova, E.P., Kurliuk, A.V., and Shakola, T.V., Int. J. Biol. Macromol., vol. 137, pp. 592–603.
Kritchenkov, A.S., Egorov, A.R., Artemjev, A.A., Kritchenkov, I.S., Volkova, O.V., Kurliuk, A.V., Shakola, T.V., Rubanik, V.V., Tskhovrebov, A.G., Yagafarov, N.Z., and Khrustalev, V.N., Int. J. Biol. Macromol., 2020, vol. 143, pp. 143–152.
Kritchenkov, A.S., Egorov, A.R., Volkova, O.V., Kritchenkov, I.S., Kurliuk, A.V., Shakola, T.V., and Khrustalev, V.N., Int. J. Biol. Macromol., 2019, vol. 139, pp. 103–113.
Semenov, K.N., Charykov, N.A., Keskinov, V.A., Kritchenkov, A.S., and Murin, I.V., Industr. Eng. Chem. Res., 2013, vol. 52, no. 46, pp. 16095–16100.
Bioassay Techniques for Drug Development, Rahman, A.-U., Choudhary, M.I., and Thomson, W.J., Eds., Harwood Acad. Publ., 2005.
Funding
This work was supported by the Program of Strategic Academic Leadership of the Peoples’ Friendship University of Russia, “Priority-2030", the Russian Foundation for Basic Research (RFBR, project nos. 20-53-04027 and 20-53-00009), the Belarusian Republican Foundation for Fundamental Research (T20R-375 and X21RM-081) within the framework of scientific projects, the Vietnam Academy of Science and Technology in the framework of scientific project no. 20-53-54006, and the RFBR in the framework of scientific projects no. 19-016-00077, 19-33-60039, and no. 20-04-60014.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Egorov, A.R., Artemjev, A.A., Kozyrev, V.A. et al. Synthesis of Selenium-Containing Chitosan Derivatives and Their Antibacterial Activity. Appl Biochem Microbiol 58, 132–135 (2022). https://doi.org/10.1134/S0003683822020053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683822020053