Abstract
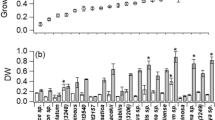
Cyanobacteria are recognized as producers of bioactive substances and phycobiliproteins, whose medicinal and functional food properties have led to increased interest in recent years. In the present study, the biomass production and phycobiliprotein content in cyanobacterial strains belonging to Anabaena, Nostoc and Spirulina genera were investigated under the conditions of continuous illumination and mixotrophic nutrition. The results showed that biomass production was strongly stimulated by continuous light in Spirulina strains (4.5-fold), and by organic carbon sources in N2-fixing strains (2.1–2.8-fold). The strategy of cells to accumulate primarily blue pigment phycocyanin and bluish green allophycocyanin was revealed under tested conditions. Furthermore, in the case of Spirulina S1 grown with glycerol, the culture medium became dense and changed its colour to pink, which may indicate the release of compounds including pigment(s) outside the cell, the phenomenon that seem to be rare among cyanobacteria. Moreover, under continuous light, in this strain the highest biomass level of 4.0 mg/mL was achieved, wherein phycocyanin and allophycocyanin content was increased 12- and 16-fold, respectively, which indicates the high potential of this strain for further investigation.
Similar content being viewed by others
References
Chisti, Y., Biotechnol. Adv., 2007, vol. 25, no. 3, pp. 294–306.
Andrade, M.R. and Costa, J.A.V., Aquaculture, 2007, vol. 264, nos. 1–4, pp. 130–134.
Kong, W., Hong, Y., Yun-Tao, C., Hao, S., Shao-Feng, H., and Chun-G., X., Food Technol. Biotechnol., 2013, vol. 51, no. 1, pp. 62–69.
Yu, H., Jia, S., and Dai, Y., J. Appl. Phycol., 2009, vol. 21, no. 1, pp. 127–133.
Bhatnagar, A., Chinnasamy, S., Singh, M., and Das, K.C., Appl. Energ., 11, vol. 88, no. 10, pp. 3425–3431.
Yu, G., Shi, D., Cai, Z., Cong, W., and Ouyang, F., Chinese J. Chem. Eng., 2011, vol. 19, no. 1, pp. 108–115.
Grossman, A.R., Bhaya, D., and He, Q., J. Biol. Chem., 2001, vol. 276, no. 15, pp. 11449–11452.
Stadnichuk, I.N. and Tropin, I.V., Appl. Biochem. Microbiol., 2017, vol. 53, no. 1, pp. 1–10.
Chaneva, G., Furnadzhieva, S., Minkova, K., and Lukavsky, J., J. Appl. Phycol., 2007, vol. 19, no. 5, pp. 537–544.
Simeunović, J., Marković, S., Kovač, D., Mišan, A., Mandić, A., and Svirčev, Z., Food Feed Res., 2012, vol. 39, pp. 23–31.
Markou, G. and Georgakakis, D., Appl. Energ., 2011, vol. 88, pp. 3389–3401.
Özyur., G., Uslu, L., Yuvka, I., Gökdogan, S., Atci, G., Ak, B., and Isik, O., J. Food Qual., 2015, vol. 38, no. 4, pp. 268–272.
Khajepour, F., Hosseini, S.A., Nasrabadi, R.G., and Markou, G., Appl. Biochem. Biotechnol., 2015, vol. 176, no. 8, pp. 2279–2289.
Prasanna, R., Pabby, A., Saxena, S., and Singh, P.K., J. Plant Physiol., 2004, vol. 161, no. 10, pp. 1125–1132.
Safafar, H., Wagenen, J., Møller, P., and Jacobsen, C., Mar. Drugs, 2015, vol. 13, no. 12, pp. 7339–7356.
Becker, E.W., Microalgae: Biotechnology and Microbiology, Cambridge: Cambridge University Press, 1994.
Simeunović, J., Bešlin, K., Svirčev, Z., Kovač, D., and Babić, O., J. Appl. Phycol., 2013, vol. 25, no. 2, pp. 597–607.
Chen, T., Zheng, W., Yang, F., Bai, Y., and Wong, Y., Enzyme Microb. Technol., 2006, vol. 39, no. 1, pp. 103–107.
Kepekçi, R.A. and Saygideger, S.D., J. Appl. Phycol., 2012, vol. 24, no. 4, pp. 897–905.
De Chaza., N.M. and Smith, G.D., Microbiology, 1994, vol. 140, pp. 3183–3189.
Sun, Y.N., Wu, D., Zhou, M., and Zhao, K.H., Acta Hydrobiol. Sin., 2004, vol. 28, no. 3, pp. 275–278.
Rippka, R., Deruelles, J., Waterbury, J.B., Herdman, M., and Stanier, R.Y., J. Gen. Microbiol., 1979, vol. 111, pp. 1–61.
Ogawa, T. and Terui, G., J. Ferment. Technol., 1970, vol. 48, pp. 361–367.
Mackinney, G., J. Biol. Chem., 1941, vol. 140, pp. 315–322.
APHA, Standard Methods for the Examination of Waste and Wastewater, Washington: American Public Health Association, 1992.
Bennett, A. and Bogorad, L., J. Cell Biol., 1973, vol. 58, no. 2, pp. 419–435.
Jacob-Lopes, E., Scoparo, C.H.G., Lacerda, L., and Franco, T.T., Chem. Eng. Process., 2009, vol. 48, no. 1, pp. 306–310.
Toxic Cyanobacteria in Water, Chorus, I. and Bartram, J., Eds., London: E. and F.N. Spon, 1999.
Ravelonandro, P.H., Ratianarivo, D.H., Joannis-Cassan, C., Isambert, A., and Raherimandimby, M., J. Chem. Technol. Biot., 2008, vol. 83, no. 6, pp. 842–848.
Feng, X., Bandyopadhyay, A., Berla, B., Page, L., Wu, B., Pakrasi, H.B., and Tang, Y.J., Microbiology, 2010, vol. 156, no. 8, pp. 2566–2574.
De Philippi., R., Sili, C., Paperi, R., and Vincenzini, M., J. Appl. Phycol., 2001, vol. 13, no. 4, pp. 293–299.
Karseno, Harada, K., Bamba, T., Dwi, S., Mahakhant, A., Yoshikawa, T., and Hirata, K., Biotechnol. Lett., 2009, vol. 31, no. 7, pp. 999–1003.
Susilaningsih, D., HAYATI J. Biosci., 2007, vol. 14, no. 1, pp. 18–22.
Shalaby, E.A. and Shanab, S.M.M., Indian J. Geomarine Sci., 2013, vol. 42, no. 5, pp. 556–564.
Babić, O., Simeunović, J., and Kovač, D., Contemp. Agr., 2014, vol. 63, no. 3, pp. 291–300.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Kovač, D., Babić, O., Milovanović, I. et al. The production of biomass and phycobiliprotein pigments in filamentous cyanobacteria: the impact of light and carbon sources. Appl Biochem Microbiol 53, 539–545 (2017). https://doi.org/10.1134/S000368381705009X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000368381705009X